Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells
- PMID: 20332205
- PMCID: PMC2851995
- DOI: 10.1073/pnas.0912437107
Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells
Abstract
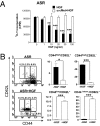
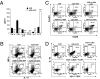
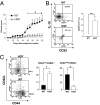
Immune-mediated diseases of the CNS, such as multiple sclerosis and its animal model, experimental autoimmune encephalitis (EAE), are characterized by the activation of antigen-presenting cells and the infiltration of autoreactive lymphocytes within the CNS, leading to demyelination, axonal damage, and neurological deficits. Hepatocyte growth factor (HGF) is a pleiotropic factor known for both neuronal and oligodendrocytic protective properties. Here, we assess the effect of a selective overexpression of HGF by neurons in the CNS of C57BL/6 mice carrying an HGF transgene (HGF-Tg mice). EAE induced either by immunization with myelin oligodendrocyte glycoprotein peptide or by adoptive transfer of T cells was inhibited in HGF-Tg mice. Notably, the level of inflammatory cells infiltrating the CNS decreased, except for CD25(+)Foxp3(+) regulatory T (T(reg)) cells, which increased. A strong T-helper cell type 2 cytokine bias was observed: IFN-gamma and IL-12p70 decreased in the spinal cord of HGF-Tg mice, whereas IL-4 and IL-10 increased. Antigen-specific response assays showed that HGF is a potent immunomodulatory factor that inhibits dendritic cell (DC) function along with differentiation of IL-10-producing T(reg) cells, a decrease in IL-17-producing T cells, and down-regulation of surface markers of T-cell activation. These effects were reversed fully when DC were pretreated with anti-cMet (HGF receptor) antibodies. Our results suggest that, by combining both potentially neuroprotective and immunomodulatory effects, HGF is a promising candidate for the development of new treatments for immune-mediated demyelinating diseases associated with neurodegeneration such as multiple sclerosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


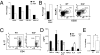
References
-
- Birchmeier C, Gherardi E. Developmental roles of HGF/SF and its receptor, the c-Met tyrosine kinase. Trends Cell Biol. 1998;8:404–410. - PubMed
-
- Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122:1450–1459. - PubMed
-
- Nakamura T, et al. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–443. - PubMed
-
- Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:239–242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

