FOXD3 is a mutant B-RAF-regulated inhibitor of G(1)-S progression in melanoma cells
- PMID: 20332228
- PMCID: PMC2848900
- DOI: 10.1158/0008-5472.CAN-09-3139
FOXD3 is a mutant B-RAF-regulated inhibitor of G(1)-S progression in melanoma cells
Abstract
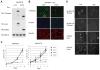
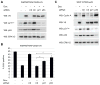
The forkhead box transcription factor FOXD3 is a stemness factor that prevents the production of melanocyte progenitors from the developing neural crest; however, its role in human cancers is not known. Transformation of melanocytes gives rise to melanoma. In two thirds of melanomas, the serine/threonine kinase B-RAF is mutated to a constitutively active form. Here, we show that FOXD3 levels are upregulated following attenuation of B-RAF and mitogen-activated protein/extracellular signal-regulated kinase (ERK) kinase (MEK) signaling in mutant B-RAF harboring human melanoma cells. This effect was selective because FOXD3 was not upregulated following MEK inhibition in wild-type B-RAF melanoma cells and mutant B-RAF thyroid carcinoma cells. Ectopic FOXD3 expression potently inhibited melanoma cell growth without altering mutant B-RAF activation of ERK1/2. Inhibition of cell growth was due to a potent G(1) cell cycle arrest and was associated with p53-dependent upregulation of p21(Cip1). FOXD3-induced cell cycle arrest was prevented by p53 depletion and, to a lesser extent, p21(Cip1) depletion. These studies show that FOXD3 is suppressed by B-RAF, uncover a novel role and mechanism for FOXD3 as a negative cell cycle regulator, and have implications for the repression of melanocytic lineage cells.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures




References
-
- Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–59. - PubMed
-
- Sutton J, Costa R, Klug M, et al. Genesis, a winged helix transcriptional repressor with expression restricted to embryonic stem cells. J Biol Chem. 1996;271:23126–33. - PubMed
-
- Thomas AJ, Erickson CA. The making of a melanocyte: the specification of melanoblasts from the neural crest. Pigment Cell Melanoma Res. 2008;21:598–610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

