Dual regulation by apurinic/apyrimidinic endonuclease-1 inhibits gastric epithelial cell apoptosis during Helicobacter pylori infection
- PMID: 20332233
- PMCID: PMC2848894
- DOI: 10.1158/0008-5472.CAN-09-4136
Dual regulation by apurinic/apyrimidinic endonuclease-1 inhibits gastric epithelial cell apoptosis during Helicobacter pylori infection
Abstract
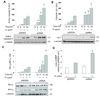
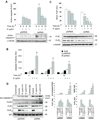
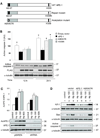
Human apurinic/apyrimidinic endonuclease-1 (APE-1), a key enzyme involved in repair of oxidative DNA base damage, is an important transcriptional coregulator. We previously reported that Helicobacter pylori infection induces apoptosis and increases APE-1 expression in human gastric epithelial cells (GEC). Although both the DNA repair activity and the acetylation-mediated transcriptional regulation of APE-1 are required to prevent cell death, the mechanisms of APE-1-mediated inhibition of infection-induced apoptosis are unclear. Here, we show that short hairpin RNA-mediated stable suppression of APE-1 results in increased apoptosis in GEC after H. pylori infection. We show that programmed cell death involves both the caspase-9-mediated mitochondrial pathway and the caspase-8-dependent extrinsic pathway by measuring different markers for both the pathways. Overexpression of wild-type APE-1 in APE-1-suppressed GEC reduced apoptosis after infection; however, overexpression of the DNA repair mutant or the nonacetylable mutant of APE-1 alone was unable to reduce apoptosis, suggesting that both DNA repair and acetylation functions of APE-1 modulate programmed cell death. We show for the first time that the DNA repair activity of APE-1 inhibits the mitochondrial pathway, whereas the acetylation function inhibits the extrinsic pathway during H. pylori infection. Thus, our findings establish that the two different functions of APE-1 differentially regulate the intrinsic and the extrinsic pathway of H. pylori-mediated GEC apoptosis. As proapoptotic and antiapoptotic mechanisms determine the development and progression of gastritis, gastric ulceration, and gastric cancer, this dual regulatory role of APE-1 represents one of the important molecular strategies by H. pylori to sustain chronic infection.
Figures



References
-
- Crowe SE. Helicobacter infection, chronic inflammation, and the development of malignancy. Curr Opin Gastroenterol. 2005;21:32–38. - PubMed
-
- Ernst PB, Peura DA, Crowe SE. The translation of Helicobacter pylori basic research to patient care. Gastroenterology. 2006;130:188–206. - PubMed
-
- Crowe SE, Alvarez L, Sherman PM, et al. Expression of interleukin-8 and CD54 by human gastric epithelium after H. pylori infection in vitro. Gastroenterology. 1995;108:65–74. - PubMed
-
- Bagchi D, Bhattacharya G, Stohs SJ. Production of reactive oxygen species by gastric cells in association with Helicobacter pylori. Free Rad Res. 1996;24:439–450. - PubMed
-
- Teshima S, Rokutan K, Nikawa T, Kishi K. Guinea pig gastric mucosal cells produce abundant superoxide anion through an NADPH oxidase-like system. Gastroenterology. 1998;115:1186–1196. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

