Akt isoforms differentially regulate neutrophil functions
- PMID: 20332370
- PMCID: PMC2879106
- DOI: 10.1182/blood-2009-11-255323
Akt isoforms differentially regulate neutrophil functions
Abstract
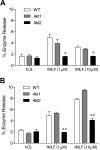
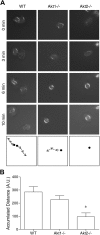
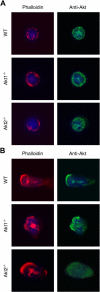
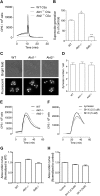
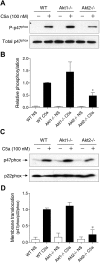
In neutrophils, the phosphoinositide 3-kinase/Akt signaling cascade is involved in migration, degranulation, and O(2)(-) production. However, it is unclear whether the Akt kinase isoforms have distinct functions in neutrophil activation. Here we report functional differences between the 2 major Akt isoforms in neutrophil activation on the basis of studies in which we used individual Akt1 and Akt2 knockout mice. Akt2(-/-) neutrophils exhibited decreased cell migration, granule enzyme release, and O(2)(-) production compared with wild-type and Akt1(-/-) neutrophils. Surprisingly, Akt2 deficiency and pharmacologic inhibition of Akt also abrogated phorbol ester-induced O(2)(-) production, which was unaffected by treatment with the phosphoinositide 3-kinase inhibitor LY294002. The decreased O(2)(-) production in Akt2(-/-) neutrophils was accompanied by reduced p47(phox) phosphorylation and its membrane translocation, suggesting that Akt2 is important for the assembly of phagocyte nicotinamide adenine dinucleotide phosphate oxidase. In wild-type neutrophils, Akt2 but not Akt1 translocated to plasma membrane upon chemoattractant stimulation and to the leading edge in polarized neutrophils. In the absence of Akt2, chemoattractant-induced Akt protein phosphorylation was significantly reduced. These results demonstrate a predominant role of Akt2 in regulating neutrophil functions and provide evidence for differential activation of the 2 Akt isoforms in neutrophils.
Figures


References
-
- Konishi H, Kuroda S, Tanaka M, et al. Molecular cloning and characterization of a new member of the RAC protein kinase family: association of the pleckstrin homology domain of three types of RAC protein kinase with protein kinase C subspecies and beta gamma subunits of G proteins. Biochem Biophys Res Commun. 1995;216(2):526–534. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

