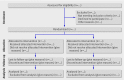
CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials
- PMID: 20332509
- PMCID: PMC2844940
- DOI: 10.1136/bmj.c332
CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials
Abstract
The CONSORT statement is used worldwide to improve the reporting of randomised controlled trials. Kenneth Schulz and colleagues describe the latest version, CONSORT 2010, which updates the reporting guideline based on new methodological evidence and accumulating experience
Conflict of interest statement
Competing interests: Uniform disclosure of potential conflicts of interest: all authors have completed the ICMJE unified competing interest form at
Figures
Comment in
-
The new CONSORT statement.BMJ. 2010 Mar 23;340:c1432. doi: 10.1136/bmj.c1432. BMJ. 2010. PMID: 20332507 No abstract available.
References
-
- Chan AW, Altman DG. Epidemiology and reporting of randomised trials published in PubMed journals. Lancet 2005;365:1159-62. - PubMed
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637-9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical