Toward gene therapy for cystic fibrosis using a lentivirus pseudotyped with Sendai virus envelopes
- PMID: 20332767
- PMCID: PMC2889732
- DOI: 10.1038/mt.2010.13
Toward gene therapy for cystic fibrosis using a lentivirus pseudotyped with Sendai virus envelopes
Abstract
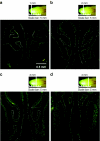
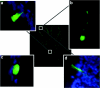
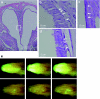
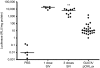
Gene therapy for cystic fibrosis (CF) is making encouraging progress into clinical trials. However, further improvements in transduction efficiency are desired. To develop a novel gene transfer vector that is improved and truly effective for CF gene therapy, a simian immunodeficiency virus (SIV) was pseudotyped with envelope proteins from Sendai virus (SeV), which is known to efficiently transduce unconditioned airway epithelial cells from the apical side. This novel vector was evaluated in mice in vivo and in vitro directed toward CF gene therapy. Here, we show that (i) we can produce relevant titers of an SIV vector pseudotyped with SeV envelope proteins for in vivo use, (ii) this vector can transduce the respiratory epithelium of the murine nose in vivo at levels that may be relevant for clinical benefit in CF, (iii) this can be achieved in a single formulation, and without the need for preconditioning, (iv) expression can last for 15 months, (v) readministration is feasible, (vi) the vector can transduce human air-liquid interface (ALI) cultures, and (vii) functional CF transmembrane conductance regulator (CFTR) chloride channels can be generated in vitro. Our data suggest that this lentiviral vector may provide a step change in airway transduction efficiency relevant to a clinical programme of gene therapy for CF.
Figures




References
-
- Pilewski JM., and , Frizzell RA. Role of CFTR in airway disease. Physiol Rev. 1999;79 1 Suppl:S215–S255. - PubMed
-
- Griesenbach U, Geddes DM., and , Alton EW. Gene therapy progress and prospects: cystic fibrosis. Gene Ther. 2006;13:1061–1067. - PubMed
-
- Yonemitsu Y, Kitson C, Ferrari S, Farley R, Griesenbach U, Judd D, et al. Efficient gene transfer to airway epithelium using recombinant Sendai virus. Nat Biotechnol. 2000;18:970–973. - PubMed
-
- Ferrari S, Griesenbach U, Shiraki-Iida T, Shu T, Hironaka T, Hou X, et al. A defective nontransmissible recombinant Sendai virus mediates efficient gene transfer to airway epithelium in vivo. Gene Ther. 2004;11:1659–1664. - PubMed
-
- Engelhardt JF. Stem cell niches in the mouse airway. Am J Respir Cell Mol Biol. 2001;24:649–652. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

