The role of the receptor for advanced glycation end-products in a murine model of silicosis
- PMID: 20333255
- PMCID: PMC2841632
- DOI: 10.1371/journal.pone.0009604
The role of the receptor for advanced glycation end-products in a murine model of silicosis
Abstract
Background: The role of the receptor for advanced glycation end-products (RAGE) has been shown to differ in two different mouse models of asbestos and bleomycin induced pulmonary fibrosis. RAGE knockout (KO) mice get worse fibrosis when challenged with asbestos, whereas in the bleomycin model they are largely protected against fibrosis. In the current study the role of RAGE in a mouse model of silica induced pulmonary fibrosis was investigated.
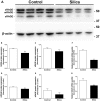
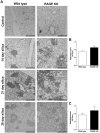
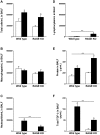
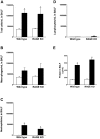
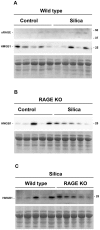
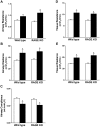
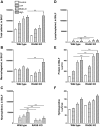
Methodology/principal findings: Wild type (WT) and RAGE KO mice received a single intratracheal (i.t.) instillation of silica in saline or saline alone as vehicle control. Fourteen days after treatment mice were subjected to a lung mechanistic study and the lungs were lavaged and inflammatory cells, protein and TGF-beta levels in lavage fluid determined. Lungs were subsequently either fixed for histology or excised for biochemical assessment of fibrosis and determination of RAGE protein- and mRNA levels. There was no difference in the inflammatory response or degree of fibrosis (hydroxyproline levels) in the lungs between WT and RAGE KO mice after silica injury. However, histologically the fibrotic lesions in the RAGE KO mice had a more diffuse alveolar septal fibrosis compared to the nodular fibrosis in WT mice. Furthermore, RAGE KO mice had a significantly higher histologic score, a measure of affected areas of the lung, compared to WT silica treated mice. A lung mechanistic study revealed a significant decrease in lung function after silica compared to control, but no difference between WT and RAGE KO. While a dose response study showed similar degrees of fibrosis after silica treatment in the two strains, the RAGE KO mice had some differences in the inflammatory response compared to WT mice.
Conclusions/significance: Aside from the difference in the fibrotic pattern, these studies showed no indicators of RAGE having an effect on the severity of pulmonary fibrosis following silica injury.
Conflict of interest statement
Figures
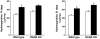
Similar articles
-
Paradoxical function for the receptor for advanced glycation end products in mouse models of pulmonary fibrosis.Int J Clin Exp Pathol. 2011 Mar;4(3):241-54. Epub 2011 Mar 21. Int J Clin Exp Pathol. 2011. PMID: 21487520 Free PMC article.
-
The role of the receptor for advanced glycation end-products in lung fibrosis.Am J Physiol Lung Cell Mol Physiol. 2007 Dec;293(6):L1427-36. doi: 10.1152/ajplung.00075.2007. Epub 2007 Oct 19. Am J Physiol Lung Cell Mol Physiol. 2007. PMID: 17951314
-
Lack of the receptor for advanced glycation end-products attenuates E. coli pneumonia in mice.PLoS One. 2011;6(5):e20132. doi: 10.1371/journal.pone.0020132. Epub 2011 May 23. PLoS One. 2011. PMID: 21629785 Free PMC article.
-
All the "RAGE" in lung disease: The receptor for advanced glycation endproducts (RAGE) is a major mediator of pulmonary inflammatory responses.Paediatr Respir Rev. 2017 Jun;23:40-49. doi: 10.1016/j.prrv.2017.03.012. Epub 2017 Mar 18. Paediatr Respir Rev. 2017. PMID: 28416135 Free PMC article. Review.
-
RAGE pathways play an important role in regulation of organ fibrosis.Life Sci. 2023 Jun 15;323:121713. doi: 10.1016/j.lfs.2023.121713. Epub 2023 Apr 23. Life Sci. 2023. PMID: 37088412 Review.
Cited by
-
RAGE Is Essential for Subretinal Fibrosis in Laser-Induced Choroidal Neovascularization: Therapeutic Implications.Invest Ophthalmol Vis Sci. 2025 Jun 2;66(6):30. doi: 10.1167/iovs.66.6.30. Invest Ophthalmol Vis Sci. 2025. PMID: 40488713 Free PMC article.
-
The complex cascade of cellular events governing inflammasome activation and IL-1β processing in response to inhaled particles.Part Fibre Toxicol. 2016 Aug 12;13(1):40. doi: 10.1186/s12989-016-0150-8. Part Fibre Toxicol. 2016. PMID: 27519871 Free PMC article. Review.
-
Paradoxical function for the receptor for advanced glycation end products in mouse models of pulmonary fibrosis.Int J Clin Exp Pathol. 2011 Mar;4(3):241-54. Epub 2011 Mar 21. Int J Clin Exp Pathol. 2011. PMID: 21487520 Free PMC article.
-
Macrophage-derived HMGB1 is dispensable for tissue fibrogenesis.FASEB Bioadv. 2019 Feb 12;1(4):227-245. doi: 10.1096/fba.2018-00035. eCollection 2019 Apr. FASEB Bioadv. 2019. PMID: 32123829 Free PMC article.
-
Receptor for advanced glycation end-products and World Trade Center particulate induced lung function loss: A case-cohort study and murine model of acute particulate exposure.PLoS One. 2017 Sep 19;12(9):e0184331. doi: 10.1371/journal.pone.0184331. eCollection 2017. PLoS One. 2017. PMID: 28926576 Free PMC article.
References
-
- Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. - PubMed
-
- Blum RH, Carter SK, Agre K. A clinical review of bleomycin–a new antineoplastic agent. Cancer. 1973;31:903–914. - PubMed
-
- The National Institute for Occupational Safety and Health N. 2008. Silicosis: Number of deaths, crude and age-adjusted death rates, U.S. residents age 15 and over, 1968–2004. Ref. 2007F03-01.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

