Cyclotraxin-B, the first highly potent and selective TrkB inhibitor, has anxiolytic properties in mice
- PMID: 20333308
- PMCID: PMC2841647
- DOI: 10.1371/journal.pone.0009777
Cyclotraxin-B, the first highly potent and selective TrkB inhibitor, has anxiolytic properties in mice
Abstract
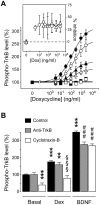
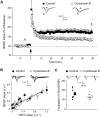
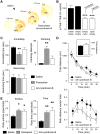
In the last decades, few mechanistically novel therapeutic agents have been developed to treat mental and neurodegenerative disorders. Numerous studies suggest that targeting BDNF and its TrkB receptor could be a promising therapeutic strategy for the treatment of brain disorders. However, the development of potent small ligands for the TrkB receptor has proven to be difficult. By using a peptidomimetic approach, we developed a highly potent and selective TrkB inhibitor, cyclotraxin-B, capable of altering TrkB-dependent molecular and physiological processes such as synaptic plasticity, neuronal differentiation and BDNF-induced neurotoxicity. Cyclotraxin-B allosterically alters the conformation of TrkB, which leads to the inhibition of both BDNF-dependent and -independent (basal) activities. Finally, systemic administration of cyclotraxin-B to mice results in TrkB inhibition in the brain with specific anxiolytic-like behavioral effects and no antidepressant-like activity. This study demonstrates that cyclotraxin-B might not only be a powerful tool to investigate the role of BDNF and TrkB in physiology and pathology, but also represents a lead compound for the development of new therapeutic strategies to treat brain disorders.
Conflict of interest statement
Figures



References
-
- Chao MV, Hempstead BL. p75 and Trk: a two-receptor system. Trends Neurosci. 1995;18:321–326. - PubMed
-
- Lee FS, Rajagopal R, Kim AH, Chang PC, Chao MV. Activation of Trk neurotrophin receptor signaling by pituitary adenylate cyclase-activating polypeptides. J Biol Chem. 2002;277:9096–9102. - PubMed
-
- Huang YZ, Pan E, Xiong ZQ, McNamara JO. Zinc-mediated transactivation of TrkB potentiates the hippocampal mossy fiber-CA3 pyramid synapse. Neuron. 2008;57:546–558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

