Validation, In-Depth Analysis, and Modification of the Micropipette Aspiration Technique
- PMID: 20333318
- PMCID: PMC2843006
- DOI: 10.1007/s12195-009-0071-9
Validation, In-Depth Analysis, and Modification of the Micropipette Aspiration Technique
Abstract
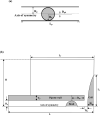
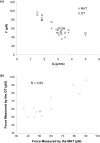
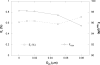
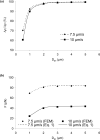
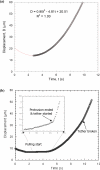
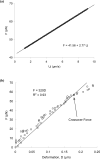
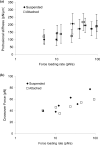
The micropipette aspiration technique (MAT) has been successfully applied to many studies in cell adhesion such as leukocyte-endothelium interactions. However, this technique has never been validated experimentally and it has been only employed to impose constant forces. In this study, we validated the force measurement of the MAT with the optical trap and analyzed two technical issues of the MAT, force-transducer offset and cell-micropipette gap, with finite element simulation. We also modified the MAT so that increasing or decreasing forces can be applied. With the modified MAT, we studied tether extraction from endothelial cells by pulling single tethers at increasing velocities and constant force loading rates. Before the onset of tether extraction, an apparently-linear surface protrusion of a few hundred nanometers was observed, which is likely related to membrane receptors pulling on the underlying cytoskeleton. The strength of the modified MAT lies in its capability and consistency to apply a wide range of force loading rates from several piconewtons per second up to thousands of piconewtons per second. With this modification, the MAT becomes more versatile in the study of single molecule and single cell biophysics.
Figures

Similar articles
-
Endothelial Surface Protrusion by a Point Force.Biophys J. 2016 Mar 8;110(5):1150-7. doi: 10.1016/j.bpj.2016.01.007. Biophys J. 2016. PMID: 26958891 Free PMC article.
-
Biomechanics of Neutrophil Tethers.Life (Basel). 2021 May 31;11(6):515. doi: 10.3390/life11060515. Life (Basel). 2021. PMID: 34073130 Free PMC article. Review.
-
Finite element analysis of imposing femtonewton forces with micropipette aspiration.Ann Biomed Eng. 2002 Apr;30(4):546-54. doi: 10.1114/1.1476017. Ann Biomed Eng. 2002. PMID: 12086005
-
Membrane tether extraction from human umbilical vein endothelial cells and its implication in leukocyte rolling.Biophys J. 2004 Nov;87(5):3561-8. doi: 10.1529/biophysj.104.047514. Epub 2004 Aug 31. Biophys J. 2004. PMID: 15339799 Free PMC article.
-
Quantifying cell-adhesion strength with micropipette manipulation: principle and application.Front Biosci. 2004 Sep 1;9:2183-91. doi: 10.2741/1386. Front Biosci. 2004. PMID: 15353280 Review.
Cited by
-
Unfolding the A2 domain of von Willebrand factor with the optical trap.Biophys J. 2010 Apr 21;98(8):1685-93. doi: 10.1016/j.bpj.2009.12.4324. Biophys J. 2010. PMID: 20409490 Free PMC article.
-
Endothelial Surface Protrusion by a Point Force.Biophys J. 2016 Mar 8;110(5):1150-7. doi: 10.1016/j.bpj.2016.01.007. Biophys J. 2016. PMID: 26958891 Free PMC article.
-
Biomechanics of Neutrophil Tethers.Life (Basel). 2021 May 31;11(6):515. doi: 10.3390/life11060515. Life (Basel). 2021. PMID: 34073130 Free PMC article. Review.
-
Characterization of cytoplasmic viscosity of hundreds of single tumour cells based on micropipette aspiration.R Soc Open Sci. 2019 Mar 20;6(3):181707. doi: 10.1098/rsos.181707. eCollection 2019 Mar. R Soc Open Sci. 2019. PMID: 31032026 Free PMC article.
-
The constitutive equation for membrane tether extraction.Ann Biomed Eng. 2010 Dec;38(12):3756-65. doi: 10.1007/s10439-010-0117-0. Epub 2010 Jul 8. Ann Biomed Eng. 2010. PMID: 20614242 Free PMC article.
References
-
- Binnig G, Quate CF, Gerber C. Atomic force microscope. Phys. Rev. Lett. 1986;56:930–933. - PubMed
-
- Bungay PM, Brenner H. The motion of a closely-fitting sphere in a fluid-filled tube. Int. J. Multiphase Flow. 1973;1:25–56.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

