Redox reactions induced by nitrosative stress mediate protein misfolding and mitochondrial dysfunction in neurodegenerative diseases
- PMID: 20333559
- PMCID: PMC4586261
- DOI: 10.1007/s12035-010-8113-9
Redox reactions induced by nitrosative stress mediate protein misfolding and mitochondrial dysfunction in neurodegenerative diseases
Abstract
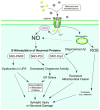
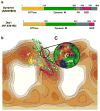
Overstimulation of N-methyl-D-aspartate (NMDA)-type glutamate receptors accounts, at least in part, for excitotoxic neuronal damage, potentially contributing to a wide range of acute and chronic neurologic diseases. Neurodegenerative disorders including Alzheimer's disease (AD) and Parkinson's disease (PD), manifest deposits of misfolded or aggregated proteins, and result from synaptic injury and neuronal death. Recent studies have suggested that nitrosative stress due to generation of excessive nitric oxide (NO) can mediate excitotoxicity in part by triggering protein misfolding and aggregation, and mitochondrial fragmentation in the absence of genetic predisposition. S-Nitrosylation, or covalent reaction of NO with specific protein thiol groups, represents a convergent signal pathway contributing to NO-induced protein misfolding and aggregation, compromised dynamics of mitochondrial fission-fusion process, thus leading to neurotoxicity. Here, we review the effect of S-nitrosylation on protein function under excitotoxic conditions, and present evidence suggesting that NO contributes to protein misfolding and aggregation via S-nitrosylating protein-disulfide isomerase or the E3 ubiquitin ligase parkin, and mitochondrial fragmentation through beta-amyloid-related S-nitrosylation of dynamin-related protein-1. Moreover, we also discuss that inhibition of excessive NMDA receptor activity by memantine, an uncompetitive/fast off-rate (UFO) drug can ameliorate excessive production of NO, protein misfolding and aggregation, mitochondrial fragmentation, and neurodegeneration.
Figures
References
-
- Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. - PubMed
-
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. - PubMed
-
- Muchowski PJ. Protein misfolding, amyloid formation, and neurodegeneration: a critical role for molecular chaperones? Neuron. 2002;35:9–12. - PubMed
-
- Emerit J, Edeas M, Bricaire F. Neurodegenerative diseases and oxidative stress. Biomed Pharmacother. 2004;58:39–46. - PubMed
-
- Beal MF. Experimental models of Parkinson’s disease. Nat Rev Neurosci. 2001;2:325–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

