The p12 subunit of human polymerase delta modulates the rate and fidelity of DNA synthesis
- PMID: 20334433
- PMCID: PMC2860660
- DOI: 10.1021/bi100042b
The p12 subunit of human polymerase delta modulates the rate and fidelity of DNA synthesis
Abstract
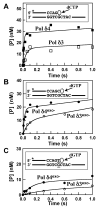
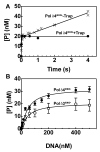
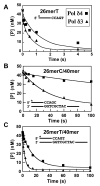
This study examines the role of the p12 subunit in the function of the human DNA polymerase delta (Pol delta) holoenzyme by comparing the kinetics of DNA synthesis and degradation catalyzed by the four-subunit complex, the three-subunit complex lacking p12, and site-directed mutants of each lacking proofreading exonuclease activity. Results show that p12 modulates the rate and fidelity of DNA synthesis by Pol delta. All four complexes synthesize DNA in a rapid burst phase and a slower, more linear phase. In the presence of p12, the burst rates of DNA synthesis are approximately 5 times faster, while the affinity of the enzyme for its DNA and dNTP substrates appears unchanged. The p12 subunit alters Pol delta fidelity by modulating the proofreading 3' to 5' exonuclease activity. In the absence of p12, Pol delta is more likely to proofread DNA synthesis because it cleaves single-stranded DNA twice as fast and transfers mismatched DNA from the polymerase to the exonuclease sites 9 times faster. Pol delta also extends mismatched primers 3 times more slowly in the absence of p12. Taken together, the changes that p12 exerts on Pol delta are ones that can modulate its fidelity of DNA synthesis. The loss of p12, which occurs in cells upon exposure to DNA-damaging agents, converts Pol delta to a form that has an increased capacity for proofreading.
Figures


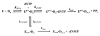
References
-
- Steitz TA. DNA polymerases: structural diversity and common mechanisms. J Biol Chem. 1999;274:17395–17398. - PubMed
-
- Johnson KA. Conformational coupling in DNA polymerase fidelity. Annu Rev Biochem. 1993;62:685–713. - PubMed
-
- Kunkel TA, Bebenek K. DNA replication fidelity. Annu Rev Biochem. 2000;69:497–529. - PubMed
-
- Doublie S, Sawaya MR, Ellenberger T. An open and closed case for all polymerases. Structure. 1999;7:R31–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

