Links between innate immunity and normal tissue radiobiology
- PMID: 20334512
- PMCID: PMC2865470
- DOI: 10.1667/RR1931.1
Links between innate immunity and normal tissue radiobiology
Abstract
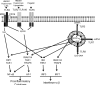
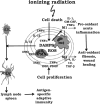
The body senses "danger" from "damaged self" molecules through members of the same receptor superfamily it uses for microbial "non-self", triggering canonical signaling pathways that lead to the generation of acute inflammatory responses. For this reason, the biology of normal tissue responses to moderate and clinically relevant doses of radiation is inextricably connected to innate immunity. The complex sequence of inflammatory events that ensues causes further cell and tissue damage to eliminate potential invaders but also leads to cytoprotective responses that limit the spread of damage and to wound healing through tissue regeneration or replacement. These sequential processes are orchestrated through multiple feedback control mechanisms involving cyclical production of free radicals and cytokines that are common to both radiation and immune signaling. This requires a concerted effort by resident tissue and inflammatory cell types, with macrophages apparently leading the way. The initial response to moderate doses of radiation therefore feeds into a pro-inflammatory paradigm whose eventual outcome is critically dependent upon the properties of the immune cells that are involved in tissue damage, regeneration and repair and that are in part under genetic influence. Importantly, these canonical pathways provide targets for interventions aimed at modifying normal tissue radiation responses. In this review, we examine areas of intersection between innate immunity and normal tissue radiobiology.
Figures

References
-
- Janeway CA., Jr. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol. Today. 1992;13:11–16. - PubMed
-
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr. A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. - PubMed
-
- Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. - PubMed
-
- Robinson MJ, Sancho D, Slack EC, LeibundGut-Landmann S, Reis e Sousa C. Myeloid C-type lectins in innate immunity. Nat. Immunol. 2006;7:1258–1265. - PubMed
-
- Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, Devera ME, Liang X, Tor M, Billiar T. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol. Rev. 2007;220:60–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

