The cytoprotective drug amifostine modifies both expression and activity of the pro-angiogenic factor VEGF-A
- PMID: 20334641
- PMCID: PMC2859403
- DOI: 10.1186/1741-7015-8-19
The cytoprotective drug amifostine modifies both expression and activity of the pro-angiogenic factor VEGF-A
Abstract
Background: Amifostine (WR-2721, delivered as Ethyol) is a phosphorylated aminothiol compound clinically used in addition to cis-platinum to reduce the toxic side effects of therapeutic treatment on normal cells without reducing their efficacy on tumour cells. Its mechanism of action is attributed to the free radical scavenging properties of its active dephosphorylated metabolite WR-1065. However, amifostine has also been described as a potent hypoxia-mimetic compound and as a strong p53 inducer; both effects are known to potently modulate vascular endothelial growth factor (VEGF-A) expression. The angiogenic properties of this drug have not been clearly defined.
Methods: Cancer cell lines and endothelial cells were used in culture and treated with Amifostine in order to study (i) the expression of angiogenesis related genes and proteins and (ii) the effects of the drug on VEGF-A induced in vitro angiogenesis.
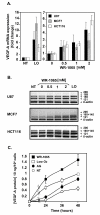
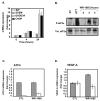
Results: We demonstrated that the treatment of several human cancer cell lines with therapeutical doses of WR-1065 led to a strong induction of different VEGF-A mRNA isoforms independently of HIF-1alpha. VEGF-A induction by WR-1065 depends on the activation of the eIF2alpha/ATF4 pathway. This up-regulation of VEGF-A mRNA was accompanied by an increased secretion of VEGF-A proteins fully active in stimulating vascular endothelial cells (EC). Nevertheless, direct treatment of EC with amifostine impaired their ability to respond to exogenous VEGF-A, an effect that correlated to the down-regulation of VEGFR-2 expression, to the reduction in cell surface binding of VEGF-A and to the decreased phosphorylation of the downstream p42/44 kinases.
Conclusions: Taken together, our results indicate that amifostine treatment modulates tumour angiogenesis by two apparently opposite mechanisms - the increased VEGF-A expression by tumour cells and the inhibition of EC capacity to respond to VEGF-A stimulation.
Figures




References
-
- News and products notes. Formulary. 1996;31:152.
-
- Shaw LM, Bonner HS, Brown DQ. Metabolic pathways of WR-2721 (ethyol, amifostine) in the BALB/c mouse. Drug Metab Dispos. 1994;22:895–902. - PubMed
-
- Smoluk GD, Fahey RC, Calabro-Jones PM, Aguilera JA, Ward JF. Radioprotection of cells in culture by WR-2721 and derivatives: form of the drug responsible for protection. Cancer Res. 1988;48:3641–3647. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

