Effectiveness of second-generation antipsychotics: a naturalistic, randomized comparison of olanzapine, quetiapine, risperidone, and ziprasidone
- PMID: 20334680
- PMCID: PMC2851682
- DOI: 10.1186/1471-244X-10-26
Effectiveness of second-generation antipsychotics: a naturalistic, randomized comparison of olanzapine, quetiapine, risperidone, and ziprasidone
Abstract
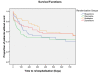
Background: No clear recommendations exist regarding which antipsychotic drug should be prescribed first for a patient suffering from psychosis. The primary aims of this naturalistic study were to assess the head-to-head effectiveness of first-line second-generation antipsychotics with regards to time until drug discontinuation, duration of index admission, time until readmission, change of psychopathology scores and tolerability outcomes.
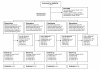
Methods: Patients >or= 18 years of age admitted to the emergency ward for symptoms of psychosis were consecutively randomized to risperidone (n = 53), olanzapine (n = 52), quetiapine (n = 50), or ziprasidone (n = 58), and followed for up to 2 years.
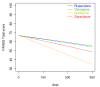
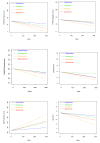
Results: A total of 213 patients were included, of which 68% were males. The sample represented a diverse population suffering from psychosis. At admittance the mean Positive and Negative Syndrome Scale (PANSS) total score was 74 points and 44% were antipsychotic drug naïve. The primary intention-to-treat analyses revealed no substantial differences between the drugs regarding the times until discontinuation of initial drug, until discharge from index admission, or until readmission. Quetiapine was superior to risperidone and olanzapine in reducing the PANSS total score and the positive subscore. Quetiapine was superior to the other drugs in decreasing the PANSS general psychopathology subscore; in decreasing the Clinical Global Impression - Severity of Illness scale score (CGI-S); and in increasing the Global Assessment of Functioning - Split version, Functions scale score (GAF-F). Ziprasidone was superior to risperidone in decreasing the PANSS positive symptoms subscore and the CGI-S score, and in increasing the GAF-F score. The drugs performed equally with regards to most tolerability outcomes except a higher increase of hip-circumference per day for olanzapine compared to risperidone, and more galactorrhoea for risperidone compared to the other groups.
Conclusions: Quetiapine appears to be a good starting drug candidate in this sample of patients admitted to hospital for symptoms of psychosis.
Trial registration: ClinicalTrials.gov ID; URL: http://www.clinicaltrials.gov/: NCT00932529.
Figures


References
-
- American Psychiatric Association. Practice Guidelines for the Treatment of Patients with Schizophrenia. Arlington, VA: American Psychiatric Association; 2004.
-
- Marder SR, Essock SM, Miller AL, Buchanan RW, Davis JM, Kane JM, Lieberman J, Schooler NR. The Mount Sinai conference on the pharmacotherapy of schizophrenia. Schiz Bull. 2002;28(1):5–16. - PubMed
-
- Moore TA, Buchanan RW, Buckley PF, Chiles JA, Conley RR, Crismon ML, Essock SM, Finnerty M, Marder SR, Miller del D, McEvoy JP, Robinson DG, Schooler NR, Shon SP, Stroup TS, Miller AL. The Texas medication algorithm project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry. 2007;68(11):1751–1762. doi: 10.4088/JCP.v68n1115. - DOI - PubMed
-
- National Institute for Clinical Excellence. Guidance on the Use of Newer (Atypical) Antipsychotic Drugs for the Treatment of Schizophrenia. Technology Appraisal Guidance--No. 2002;43 http://www.nice.org.uk/ Accessed November 02, 2008.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

