Myxoma and vaccinia viruses exploit different mechanisms to enter and infect human cancer cells
- PMID: 20334889
- PMCID: PMC2862966
- DOI: 10.1016/j.virol.2010.02.027
Myxoma and vaccinia viruses exploit different mechanisms to enter and infect human cancer cells
Abstract
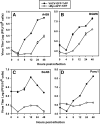
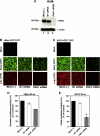
Myxoma (MYXV) and vaccinia (VACV) viruses have recently emerged as potential oncolytic agents that can infect and kill different human cancer cells. Although both are structurally similar, it is unknown whether the pathway(s) used by these poxviruses to enter and cause oncolysis in cancer cells are mechanistically similar. Here, we compared the entry of MYXV and VACV-WR into various human cancer cells and observed significant differences: 1--low-pH treatment accelerates fusion-mediated entry of VACV but not MYXV, 2--the tyrosine kinase inhibitor genistein inhibits entry of VACV, but not MYXV, 3--knockdown of PAK1 revealed that it is required for a late stage event downstream of MYXV entry into cancer cells, whereas PAK1 is required for VACV entry into the same target cells. These results suggest that VACV and MYXV exploit different mechanisms to enter into human cancer cells, thus providing some rationale for their divergent cancer cell tropisms.
2010 Elsevier Inc. All rights reserved.
Figures






References
-
- Andres A, Donovan SM, Kuhlenschmidt TB, Kuhlenschmidt MS. Isoflavones at concentrations present in soy infant formula inhibit rotavirus infection in vitro. J Nutr. 2007;137:2068–73. - PubMed
-
- Barrett JW, Alston LR, Wang F, Stanford MM, Gilbert PA, Gao X, Jimenez J, Villeneuve D, Forsyth P, McFadden G. Identification of host range mutants of myxoma virus with altered oncolytic potential in human glioma cells. J. Neurovirol. 2007a;13:549–560. - PubMed
-
- Barrett JW, Sypula J, Wang F, Alston LR, Shao Z, Gao X, Irvine TS, McFadden G. Myxoma virus M063R is a host range gene essential for virus replication in rabbit cells. Virology. 2007b;361:123–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

