Structural and biochemical characterization of peroxiredoxin Qbeta from Xylella fastidiosa: catalytic mechanism and high reactivity
- PMID: 20335172
- PMCID: PMC2871474
- DOI: 10.1074/jbc.M109.094839
Structural and biochemical characterization of peroxiredoxin Qbeta from Xylella fastidiosa: catalytic mechanism and high reactivity
Abstract
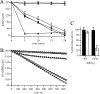
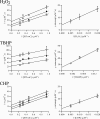
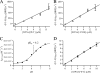
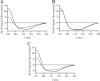
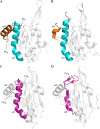
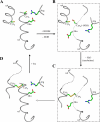
The phytopathogenic bacterium Xylella fastidiosa is the etiological agent of various plant diseases. To survive under oxidative stress imposed by the host, microorganisms express antioxidant proteins, including cysteine-based peroxidases named peroxiredoxins. This work is a comprehensive analysis of the catalysis performed by PrxQ from X. fastidiosa (XfPrxQ) that belongs to a peroxiredoxin class still poorly characterized and previously considered as moderately reactive toward hydroperoxides. Contrary to these assumptions, our competitive kinetics studies have shown that the second-order rate constants of the peroxidase reactions of XfPrxQ with hydrogen peroxide and peroxynitrite are in the order of 10(7) and 10(6) M(-1) S(-1), respectively, which are as fast as the most efficient peroxidases. The XfPrxQ disulfides were only slightly reducible by dithiothreitol; therefore, the identification of a thioredoxin system as the probable biological reductant of XfPrxQ was a relevant finding. We also showed by site-specific mutagenesis and mass spectrometry that an intramolecular disulfide bond between Cys-47 and Cys-83 is generated during the catalytic cycle. Furthermore, we elucidated the crystal structure of XfPrxQ C47S in which Ser-47 and Cys-83 lie approximately 12.3 A apart. Therefore, significant conformational changes are required for disulfide bond formation. In fact, circular dichroism data indicated that there was a significant redox-dependent unfolding of alpha-helices, which is probably triggered by the peroxidatic cysteine oxidation. Finally, we proposed a model that takes data from this work as well data as from the literature into account.
Figures



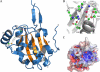
Similar articles
-
Kinetics of peroxiredoxins and their role in the decomposition of peroxynitrite.Subcell Biochem. 2007;44:83-113. doi: 10.1007/978-1-4020-6051-9_5. Subcell Biochem. 2007. PMID: 18084891 Review.
-
Structural insights on the efficient catalysis of hydroperoxide reduction by Ohr: Crystallographic and molecular dynamics approaches.PLoS One. 2018 May 21;13(5):e0196918. doi: 10.1371/journal.pone.0196918. eCollection 2018. PLoS One. 2018. PMID: 29782551 Free PMC article.
-
Catalytic mechanism of thiol peroxidase from Escherichia coli. Sulfenic acid formation and overoxidation of essential CYS61.J Biol Chem. 2003 Mar 14;278(11):9203-11. doi: 10.1074/jbc.M209888200. Epub 2003 Jan 3. J Biol Chem. 2003. PMID: 12514184 Free PMC article.
-
PrxQ B from Mycobacterium tuberculosis is a monomeric, thioredoxin-dependent and highly efficient fatty acid hydroperoxide reductase.Free Radic Biol Med. 2016 Dec;101:249-260. doi: 10.1016/j.freeradbiomed.2016.10.005. Epub 2016 Oct 15. Free Radic Biol Med. 2016. PMID: 27751911
-
Bacterial defenses against oxidants: mechanistic features of cysteine-based peroxidases and their flavoprotein reductases.Arch Biochem Biophys. 2005 Jan 1;433(1):240-54. doi: 10.1016/j.abb.2004.09.006. Arch Biochem Biophys. 2005. PMID: 15581580 Review.
Cited by
-
Mapping the active site helix-to-strand conversion of CxxxxC peroxiredoxin Q enzymes.Biochemistry. 2012 Sep 25;51(38):7638-50. doi: 10.1021/bi301017s. Epub 2012 Sep 14. Biochemistry. 2012. PMID: 22928725 Free PMC article.
-
Catalytic Thr or Ser Residue Modulates Structural Switches in 2-Cys Peroxiredoxin by Distinct Mechanisms.Sci Rep. 2016 Sep 15;6:33133. doi: 10.1038/srep33133. Sci Rep. 2016. PMID: 27629822 Free PMC article.
-
Peroxiredoxin Catalysis at Atomic Resolution.Structure. 2016 Oct 4;24(10):1668-1678. doi: 10.1016/j.str.2016.07.012. Epub 2016 Sep 1. Structure. 2016. PMID: 27594682 Free PMC article.
-
A thioredoxin-dependent peroxiredoxin Q from Corynebacterium glutamicum plays an important role in defense against oxidative stress.PLoS One. 2018 Feb 13;13(2):e0192674. doi: 10.1371/journal.pone.0192674. eCollection 2018. PLoS One. 2018. PMID: 29438446 Free PMC article.
-
A 14.7 kDa protein from Francisella tularensis subsp. novicida (named FTN_1133), involved in the response to oxidative stress induced by organic peroxides, is not endowed with thiol-dependent peroxidase activity.PLoS One. 2014 Jun 24;9(6):e99492. doi: 10.1371/journal.pone.0099492. eCollection 2014. PLoS One. 2014. PMID: 24959833 Free PMC article.
References
-
- Simpson A. J., Reinach F. C., Arruda P., Abreu F. A., Acencio M., Alvarenga R., Alves L. M., Araya J. E., Baia G. S., Baptista C. S., Barros M. H., Bonaccorsi E. D., Bordin S., Bové J. M., Briones M. R., Bueno M. R., Camargo A. A., Camargo L. E., Carraro D. M., Carrer H., Colauto N. B., Colombo C., Costa F. F., Costa M. C., Costa-Neto C. M., Coutinho L. L., Cristofani M., Dias-Neto E., Docena C., El-Dorry H., Facincani A. P., Ferreira A. J., Ferreira V. C., Ferro J. A., Fraga J. S., França S. C., Franco M. C., Frohme M., Furlan L. R., Garnier M., Goldman G. H., Goldman M. H., Gomes S. L., Gruber A., Ho P. L., Hoheisel J. D., Junqueira M. L., Kemper E. L., Kitajima J. P., Krieger J. E., Kuramae E. E., Laigret F., Lambais M. R., Leite L. C., Lemos E. G., Lemos M. V., Lopes S. A., Lopes C. R., Machado J. A., Machado M. A., Madeira A. M., Madeira H. M., Marino C. L., Marques M. V., Martins E. A., Martins E. M., Matsukuma A. Y., Menck C. F., Miracca E. C., Miyaki C. Y., Monteriro-Vitorello C. B., Moon D. H., Nagai M. A., Nascimento A. L., Netto L. E., Nhani A., Jr., Nobrega F. G., Nunes L. R., Oliveira M. A., de Oliveira M. C., de Oliveira R. C., Palmieri D. A., Paris A., Peixoto B. R., Pereira G. A., Pereira H. A., Jr., Pesquero J. B., Quaggio R. B., Roberto P. G., Rodrigues V., de M, Rosa A. J., de Rosa V. E., Jr., de Sá R. G., Santelli R. V., Sawasaki H. E., da Silva A. C., da Silva A. M., da Silva F. R., da Silva W. A., Jr., da Silveira J. F., Silvestri M. L., Siqueira W. J., de Souza A. A., de Souza A. P., Terenzi M. F., Truffi D., Tsai S. M., Tsuhako M. H., Vallada H., Van Sluys M. A., Verjovski-Almeida S., Vettore A. L., Zago M. A., Zatz M., Meidanis J., Setubal J. C. (2000) Nature 406, 151–159 - PubMed
-
- Amaro A. A., Maia M. L., Gonzales M. A. (1998) in Citrus Variegated Chlorosis (Donadio L. C., Moreira C. S. eds) pp. 123–139, Estação Experimental de Citricultura, Bebedouro, SP, Brazil
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

