Hormone binding and co-regulator binding to the glucocorticoid receptor are allosterically coupled
- PMID: 20335180
- PMCID: PMC2865338
- DOI: 10.1074/jbc.M110.108118
Hormone binding and co-regulator binding to the glucocorticoid receptor are allosterically coupled
Abstract
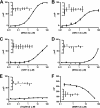
The glucocorticoid receptor initiates the cellular response to glucocorticoid steroid hormones in vertebrates. Co-regulator proteins dock to the receptor in response to hormone binding and potentiate the transcriptional activity of the receptor by modifying DNA and recruiting essential transcription factors like RNA polymerase II. Hormones and co-regulators bind at distinct sites in the ligand binding domain yet function cooperatively to mediate transcriptional control. This study reveals and quantifies energetic coupling between two binding sites using purified components. Using a library of peptides taken from co-regulator proteins, we determine the pattern of co-regulator binding to the glucocorticoid receptor ligand binding domain. We show that peptides from co-regulators differ in their effects on hormone binding and kinetics. Peptides from DAX1 and SRC1 bind with similar affinity, but DAX1 binding is coupled to hormone binding, and SRC1 is not. Mechanistic details of co-regulator binding and coupling to the hormone binding pocket are uncovered by analysis of properties endowed by mutation of a key residue in the allosteric network connecting the sites.
Figures

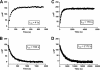

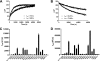
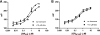

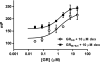

Similar articles
-
Glucocorticoid receptor ligand binding domain is sufficient for the modulation of glucocorticoid induction properties by homologous receptors, coactivator transcription intermediary factor 2, and Ubc9.Mol Endocrinol. 2005 Feb;19(2):290-311. doi: 10.1210/me.2004-0134. Epub 2004 Nov 11. Mol Endocrinol. 2005. PMID: 15539428
-
Distinct interaction of cortivazol with the ligand binding domain confers glucocorticoid receptor specificity: cortivazol is a specific ligand for the glucocorticoid receptor.J Biol Chem. 2002 Feb 15;277(7):5529-40. doi: 10.1074/jbc.M107946200. Epub 2001 Dec 10. J Biol Chem. 2002. PMID: 11741935
-
Specific DNA binding of Stat5, but not of glucocorticoid receptor, is required for their functional cooperation in the regulation of gene transcription.Mol Cell Biol. 1997 Nov;17(11):6708-16. doi: 10.1128/MCB.17.11.6708. Mol Cell Biol. 1997. PMID: 9343435 Free PMC article.
-
Glucocorticoid receptors.Monogr Endocrinol. 1979;12:49-77. doi: 10.1007/978-3-642-81265-1_3. Monogr Endocrinol. 1979. PMID: 386089 Review.
-
Mechanisms of glucocorticoid hormone action.J Steroid Biochem. 1984 Jan;20(1):77-88. doi: 10.1016/0022-4731(84)90192-4. J Steroid Biochem. 1984. PMID: 6368989 Review.
Cited by
-
The multivalency of the glucocorticoid receptor ligand-binding domain explains its manifold physiological activities.Nucleic Acids Res. 2022 Dec 9;50(22):13063-13082. doi: 10.1093/nar/gkac1119. Nucleic Acids Res. 2022. PMID: 36464162 Free PMC article.
-
Corticosterone-mediated regulation and functions of miR-218-5p in rat brain.Sci Rep. 2022 Jan 7;12(1):194. doi: 10.1038/s41598-021-03863-y. Sci Rep. 2022. PMID: 34996981 Free PMC article.
-
Single-molecule force spectroscopy reveals folding steps associated with hormone binding and activation of the glucocorticoid receptor.Proc Natl Acad Sci U S A. 2018 Nov 13;115(46):11688-11693. doi: 10.1073/pnas.1807618115. Epub 2018 Oct 26. Proc Natl Acad Sci U S A. 2018. PMID: 30366952 Free PMC article.
-
Principles of allosteric interactions in cell signaling.J Am Chem Soc. 2014 Dec 24;136(51):17692-701. doi: 10.1021/ja510028c. Epub 2014 Dec 15. J Am Chem Soc. 2014. PMID: 25474128 Free PMC article. Review.
-
Glucocorticoid receptor function regulated by coordinated action of the Hsp90 and Hsp70 chaperone cycles.Cell. 2014 Jun 19;157(7):1685-97. doi: 10.1016/j.cell.2014.04.038. Cell. 2014. PMID: 24949977 Free PMC article.
References
-
- Katzenellenbogen B. S. (1980) Annu. Rev. Physiol. 42, 17–35 - PubMed
-
- Chrousos G. P., Kino T. (2007) Stress 10, 213–219 - PubMed
-
- Rhen T., Cidlowski J. A. (2005) N. Engl. J. Med. 353, 1711–1723 - PubMed
-
- Chrousos G. P., Charmandari E., Kino T. (2004) J. Clin. Endocrinol. Metab. 89, 563–564 - PubMed
-
- Pratt W. B., Dittmar K. D. (1998) Trends Endocrinol. Metab. 9, 244–252 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

