Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections
- PMID: 20335250
- PMCID: PMC2876583
- DOI: 10.1128/JVI.00272-10
Lambda interferon renders epithelial cells of the respiratory and gastrointestinal tracts resistant to viral infections
Abstract
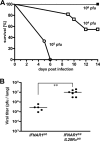
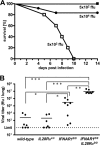
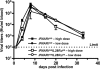
Virus-infected cells secrete a broad range of interferons (IFN) which confer resistance to yet uninfected cells by triggering the synthesis of antiviral factors. The relative contributions of the various IFN subtypes to innate immunity against virus infections remain elusive. IFN-alpha, IFN-beta, and other type I IFN molecules signal through a common, universally expressed cell surface receptor, whereas type III IFN (IFN-lambda) uses a distinct cell-type-specific receptor complex for signaling. Using mice lacking functional receptors for type I IFN, type III IFN, or both, we found that IFN-lambda plays an important role in the defense against several human pathogens that infect the respiratory tract, such as influenza A virus, influenza B virus, respiratory syncytial virus, human metapneumovirus, and severe acute respiratory syndrome (SARS) coronavirus. These viruses were more pathogenic and replicated to higher titers in the lungs of mice lacking both IFN receptors than in mice with single IFN receptor defects. In contrast, Lassa fever virus, which infects via the respiratory tract but primarily replicates in the liver, was not influenced by the IFN-lambda receptor defect. Careful analysis revealed that expression of functional IFN-lambda receptor complexes in the lung and intestinal tract is restricted to epithelial cells and a few other, undefined cell types. Interestingly, we found that SARS coronavirus was present in feces from infected mice lacking receptors for both type I and type III IFN but not in those from mice lacking single receptors, supporting the view that IFN-lambda contributes to the control of viral infections in epithelial cells of both respiratory and gastrointestinal tracts.
Figures

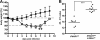
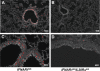

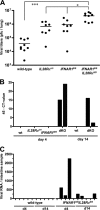
References
-
- Ank, N., M. B. Iversen, C. Bartholdy, P. Staeheli, R. Hartmann, U. B. Jensen, F. Dagnaes-Hansen, A. R. Thomsen, Z. Chen, H. Haugen, K. Klucher, and S. R. Paludan. 2008. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J. Immunol. 180:2474-2485. - PubMed
-
- Bartlett, N. W., K. Buttigieg, S. V. Kotenko, and G. L. Smith. 2005. Murine interferon lambdas (type III interferons) exhibit potent antiviral activity in vivo in a poxvirus infection model. J. Gen. Virol. 86:1589-1596. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

