Cellular and viral control over the initial events of human cytomegalovirus experimental latency in CD34+ cells
- PMID: 20335255
- PMCID: PMC2876595
- DOI: 10.1128/JVI.00348-10
Cellular and viral control over the initial events of human cytomegalovirus experimental latency in CD34+ cells
Abstract
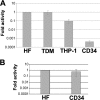
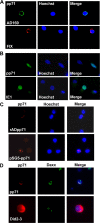
Human cytomegalovirus (HCMV) persists for the life of its host by establishing a latent infection. The identification of viral and cellular determinants of latency is the first step toward developing antiviral treatments that target and might clear or control the reservoir of latent virus. HCMV latency is established in CD34(+) cells when expression of viral immediate early (IE) proteins that initiate lytic infection is silenced. Viral IE gene expression during lytic infection is controlled by a cellular intrinsic immune defense mediated by promyelocytic leukemia nuclear body (PML-NB) proteins such as Daxx and histone deacetylases (HDACs). This defense is inactivated at the start of lytic infection by the HCMV virion tegument protein pp71, which upon viral entry traffics to the nucleus and induces Daxx degradation. Here we show that a similar defense is present, active, and not neutralized during experimental latency in CD34(+) cells infected in vitro because tegument-delivered pp71 remains in the cytoplasm. Artificial inactivation of this defense by HDAC inhibition or Daxx knockdown rescues viral IE gene expression upon infection of CD34(+) cells with a laboratory-adapted viral strain but not with clinical strains. Interestingly, coinfection of CD34(+) cells with clinical viral strains blocked the ability of an HDAC inhibitor to activate IE1 and early protein expression during infection with a laboratory-adapted strain. This suggests that in addition to the intrinsic defense, HCMV clinical strains contribute an HDAC-independent, trans-acting dominant means of control over viral gene expression during the early stages of experimental HCMV latency modeled in vitro in CD34(+) cells.
Figures




Similar articles
-
Myeloblastic cell lines mimic some but not all aspects of human cytomegalovirus experimental latency defined in primary CD34+ cell populations.J Virol. 2013 Sep;87(17):9802-12. doi: 10.1128/JVI.01436-13. Epub 2013 Jul 3. J Virol. 2013. PMID: 23824798 Free PMC article.
-
Human cytomegalovirus gene expression is silenced by Daxx-mediated intrinsic immune defense in model latent infections established in vitro.J Virol. 2007 Sep;81(17):9109-20. doi: 10.1128/JVI.00827-07. Epub 2007 Jun 27. J Virol. 2007. PMID: 17596307 Free PMC article.
-
Nuclear localization of tegument-delivered pp71 in human cytomegalovirus-infected cells is facilitated by one or more factors present in terminally differentiated fibroblasts.J Virol. 2010 Oct;84(19):9853-63. doi: 10.1128/JVI.00500-10. Epub 2010 Aug 4. J Virol. 2010. PMID: 20686028 Free PMC article.
-
Sleepless latency of human cytomegalovirus.Med Microbiol Immunol. 2015 Jun;204(3):421-9. doi: 10.1007/s00430-015-0401-6. Epub 2015 Mar 14. Med Microbiol Immunol. 2015. PMID: 25772624 Free PMC article. Review.
-
Chromatin-mediated regulation of cytomegalovirus gene expression.Virus Res. 2011 May;157(2):134-43. doi: 10.1016/j.virusres.2010.09.019. Epub 2010 Sep 25. Virus Res. 2011. PMID: 20875471 Free PMC article. Review.
Cited by
-
Heterologous viral promoters incorporated into the human cytomegalovirus genome are silenced during experimental latency.J Virol. 2013 Sep;87(17):9886-94. doi: 10.1128/JVI.01726-13. Epub 2013 Jul 3. J Virol. 2013. PMID: 23824803 Free PMC article.
-
Contribution of the Major ND10 Proteins PML, hDaxx and Sp100 to the Regulation of Human Cytomegalovirus Latency and Lytic Replication in the Monocytic Cell Line THP-1.Viruses. 2015 Jun 5;7(6):2884-907. doi: 10.3390/v7062751. Viruses. 2015. PMID: 26057166 Free PMC article.
-
The retinoblastoma tumor suppressor promotes efficient human cytomegalovirus lytic replication.J Virol. 2015 May;89(9):5012-21. doi: 10.1128/JVI.00175-15. Epub 2015 Feb 18. J Virol. 2015. PMID: 25694602 Free PMC article.
-
Epigenetic reprogramming of host and viral genes by Human Cytomegalovirus infection in Kasumi-3 myeloid progenitor cells at early times post-infection.J Virol. 2021 May 10;95(11):e00183-21. doi: 10.1128/JVI.00183-21. Epub 2021 Mar 17. J Virol. 2021. PMID: 33731453 Free PMC article.
-
Human cytomegalovirus tegument proteins (pp65, pp71, pp150, pp28).Virol J. 2012 Jan 17;9:22. doi: 10.1186/1743-422X-9-22. Virol J. 2012. PMID: 22251420 Free PMC article. Review.
References
-
- Cheung, A. K., A. Abendroth, A. L. Cunningham, and B. Slobedman. 2006. Viral gene expression during the establishment of human cytomegalovirus latent infection in myeloid progenitor cells. Blood 108:3691-3699. - PubMed
-
- Cheung, A. K., D. J. Gottlieb, B. Plachter, S. Pepperl-Klindworth, S. Avdic, A. L. Cunningham, A. Abendroth, and B. Slobedman. 2009. The role of the human cytomegalovirus UL111A gene in down-regulating CD4+ T-cell recognition of latently infected cells: implications for virus elimination during latency. Blood 114:4128-4137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

