Increased ANG II sensitivity following recovery from acute kidney injury: role of oxidant stress in skeletal muscle resistance arteries
- PMID: 20335375
- PMCID: PMC2886705
- DOI: 10.1152/ajpregu.00448.2009
Increased ANG II sensitivity following recovery from acute kidney injury: role of oxidant stress in skeletal muscle resistance arteries
Abstract
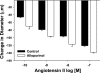
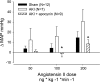
Ischemia-reperfusion (I/R)-induced acute kidney injury (AKI) results in prolonged impairment of peripheral (i.e., nonrenal) vascular function since skeletal muscle resistance arteries derived from rats 5 wk post-I/R injury, show enhanced responses to ANG II stimulation but not other constrictors. Because vascular superoxide increases ANG II sensitivity, we hypothesized that peripheral responsiveness following recovery from AKI was attributable to vascular oxidant stress. Gracilis arteries (GA) isolated from post-I/R rats (approximately 5 wk recovery) showed significantly greater superoxide levels relative to sham-operated controls, as detected by dihydroeithidium, which was further augmented by acute ANG II stimulation in vitro. Hydrogen peroxide measured by dichlorofluorescein was not affected by ANG II. GA derived from postischemic animals manifested significantly greater constrictor responses in vitro to ANG II than GA from sham-operated controls. The addition of the superoxide scavenging reagent Tempol (10(-5) M) normalized the response to values similar to sham-operated controls. Apocynin (10(-6) M) and endothelial denudation nearly abrogated all ANG II-stimulated constrictor activity in GA from post-AKI rats, suggesting an important role for an endothelial-derived source of peripheral oxidative stress. Apocynin treatment in vivo abrogated GA oxidant stress and attenuated ANG II-induced pressor responses post-AKI. Interestingly, gene expression studies in GA vessels indicated a paradoxical reduction in NADPH oxidase subunit and AT(1)-receptor genes and no effect on several antioxidant genes. Taken together, this study demonstrates that AKI alters peripheral vascular responses by increasing oxidant stress, likely in the endothelium, via an undefined mechanism.
Figures


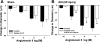
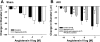
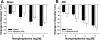

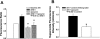
References
-
- Adler S, Huang H. Oxidant stress in kidneys of spontaneously hypertensive rats involves both oxidase overexpression and loss of extracellular superoxide dismutase. Am J Physiol Renal Physiol 287: F907–F913, 2004 - PubMed
-
- Askenazi D, Feig D, Graham N, Hui-Stickle S, Goldstein SL. 3–5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 69: 184–189, 2006 - PubMed
-
- Basile DP, Donohoe DL, Phillips SA, Frisbee JC. Enhanced skeletal muscle arteriolar reactivity to ANG II following recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol 289: R1770–R1776, 2005 - PubMed
-
- Basile DP, Donohoe DL, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001 - PubMed
-
- Basile DP, Fredrich K, Alausa MT, Vio C, Liang M, Greene AL, Cowley AW., Jr Identification of persistently altered gene expression in kidney following functional recovery from ischemic acute renal failure. Am J Physiol Regul Integr Comp Physiol 288: R953–R963, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

