Isolation and characterization of the Z-ISO gene encoding a missing component of carotenoid biosynthesis in plants
- PMID: 20335404
- PMCID: PMC2862425
- DOI: 10.1104/pp.110.153916
Isolation and characterization of the Z-ISO gene encoding a missing component of carotenoid biosynthesis in plants
Abstract
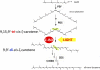
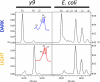
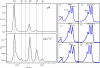
Metabolic engineering of plant carotenoids in food crops has been a recent focus for improving human health. Pathway manipulation is predicated on comprehensive knowledge of this biosynthetic pathway, which has been extensively studied. However, there existed the possibility of an additional biosynthetic step thought to be dispensable because it could be compensated for by light. This step, mediated by a putative Z-ISO, was predicted to occur in the sequence of redox reactions that are coupled to an electron transport chain and convert the colorless 15-cis-phytoene to the red-colored all-trans-lycopene. The enigma of carotenogenesis in the absence of light (e.g. in endosperm, a target for improving nutritional content) argued for Z-ISO as a pathway requirement. Therefore, understanding of plant carotenoid biosynthesis was obviously incomplete. To prove the existence of Z-ISO, maize (Zea mays) and Arabidopsis (Arabidopsis thaliana) mutants were isolated and the gene identified. Functional testing of the gene product in Escherichia coli showed isomerization of the 15-cis double bond in 9,15,9'-tri-cis-zeta-carotene, proving that Z-ISO encoded the missing step. Z-ISO was found to be important for both light-exposed and "dark" tissues. Comparative genomics illuminated the origin of Z-ISO found throughout higher and lower plants, algae, diatoms, and cyanobacteria. Z-ISO evolved from an ancestor related to the NnrU (for nitrite and nitric oxide reductase U) gene required for bacterial denitrification, a pathway that produces nitrogen oxides as alternate electron acceptors for anaerobic growth. Therefore, plant carotenogenesis evolved by recruitment of genes from noncarotenogenic bacteria.
Figures

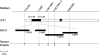




Similar articles
-
Control of carotenoid biosynthesis through a heme-based cis-trans isomerase.Nat Chem Biol. 2015 Aug;11(8):598-605. doi: 10.1038/nchembio.1840. Epub 2015 Jun 15. Nat Chem Biol. 2015. PMID: 26075523 Free PMC article.
-
Oxygenic Phototrophs Need ζ-Carotene Isomerase (Z-ISO) for Carotene Synthesis: Functional Analysis in Arthrospira and Euglena.Plant Cell Physiol. 2020 Feb 1;61(2):276-282. doi: 10.1093/pcp/pcz192. Plant Cell Physiol. 2020. PMID: 31593237
-
Maize Y9 encodes a product essential for 15-cis-zeta-carotene isomerization.Plant Physiol. 2007 Jun;144(2):1181-9. doi: 10.1104/pp.107.098996. Epub 2007 Apr 13. Plant Physiol. 2007. PMID: 17434985 Free PMC article.
-
[Advances in phytoene dehydrogenase - A review].Wei Sheng Wu Xue Bao. 2016 Nov 4;56(11):1680-90. Wei Sheng Wu Xue Bao. 2016. PMID: 29741830 Review. Chinese.
-
Carotenoids, versatile components of oxygenic photosynthesis.Prog Lipid Res. 2013 Oct;52(4):539-61. doi: 10.1016/j.plipres.2013.07.001. Epub 2013 Jul 26. Prog Lipid Res. 2013. PMID: 23896007 Review.
Cited by
-
Synechocystis sp. PCC 6803 CruA (sll0147) encodes lycopene cyclase and requires bound chlorophyll a for activity.Photosynth Res. 2017 Mar;131(3):267-280. doi: 10.1007/s11120-016-0316-0. Epub 2016 Oct 14. Photosynth Res. 2017. PMID: 27743323
-
Tissue-Specific Apocarotenoid Glycosylation Contributes to Carotenoid Homeostasis in Arabidopsis Leaves.Plant Physiol. 2015 Aug;168(4):1550-62. doi: 10.1104/pp.15.00243. Epub 2015 Jul 1. Plant Physiol. 2015. PMID: 26134165 Free PMC article.
-
Control of carotenoid biosynthesis through a heme-based cis-trans isomerase.Nat Chem Biol. 2015 Aug;11(8):598-605. doi: 10.1038/nchembio.1840. Epub 2015 Jun 15. Nat Chem Biol. 2015. PMID: 26075523 Free PMC article.
-
Perturbations in the Carotenoid Biosynthesis Pathway in Tomato Fruit Reactivate the Leaf-Specific Phytoene Synthase 2.Front Plant Sci. 2022 Feb 25;13:844748. doi: 10.3389/fpls.2022.844748. eCollection 2022. Front Plant Sci. 2022. PMID: 35283915 Free PMC article.
-
Characterization of phytoene synthases from cassava and their involvement in abiotic stress-mediated responses.Planta. 2010 Oct;232(5):1251-62. doi: 10.1007/s00425-010-1250-6. Epub 2010 Aug 25. Planta. 2010. PMID: 20737168
References
-
- Bachmann MD, Robertson DS, Bowen CC, Anderson IC. (1973) Chloroplast ultrastructure in pigment-deficient mutants of Zea mays under reduced light. J Ultrastruct Res 45: 384–406 - PubMed
-
- Bartley GE, Scolnik PA, Beyer P. (1999) Two Arabidopsis thaliana carotene desaturases, phytoene desaturase and zeta-carotene desaturase, expressed in Escherichia coli, catalyze a poly-cis pathway to yield pro-lycopene. Eur J Biochem 259: 396–403 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

