Gap junctions/hemichannels modulate interkinetic nuclear migration in the forebrain precursors
- PMID: 20335455
- PMCID: PMC2861434
- DOI: 10.1523/JNEUROSCI.4187-09.2010
Gap junctions/hemichannels modulate interkinetic nuclear migration in the forebrain precursors
Abstract
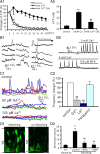
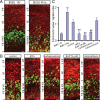
During mitotic division in the telencephalic proliferative ventricular zone (VZ), the nuclei of the neural precursors move basally away from the ventricular surface for DNA synthesis, and apically return to the surface for mitotic division; a process known as interkinetic migration or "to-and-fro" nuclear translocation. The cell, which remains attached to the ventricular surface, either continues cycling, or exits the cycle and migrates to the subventricular zone or the developing cortical plate. Although gap junctions/hemichannels are known to modulate DNA synthesis via Ca(2+) waves, the role of Ca(+) oscillations and the mechanism of nuclear translocation in the VZ precursors are unclear. Here, we provide evidence that, during apical nuclear migration, VZ precursors display dynamic spontaneous Ca(2+) transients, which depend on functional gap junctions/hemichannels via ATP release and Ca(2+)-mobilizing messenger diffusion. Furthermore, we found that blocking gap junctions/hemichannels or short hairpin RNA-mediated knockdown of Cx43 (connexin 43) retards the apically directed interkinetic nuclear migration accompanied with changes in the nuclear length/width ratio. In addition, we demonstrated that blocking functional gap junctions/hemichannels induces phosphorylation of small GTPase cdc42 in the VZ precursors. The basal phase of interkinetic migration is much slower and appears to be mediated passively by mechanical forces after cell division. Our findings indicate that functional interference with gap junctions/hemichannels during embryonic development may lead to abnormal corticogenesis and dysfunction of the cerebral cortex in adult organisms.
Figures







References
-
- Alexandre H, Van Cauwenberge A, Mulnard J. Involvement of microtubules and microfilaments in the control of the nuclear movement during maturation of mouse oocyte. Dev Biol. 1989;136:311–320. - PubMed
-
- Algan O, Rakic P. Radiation-induced, lamina-specific deletion of neurons in the primate visual cortex. J Comp Neurol. 1997;381:335–352. - PubMed
-
- Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci U S A. 2008;105:18770–18775. - PMC - PubMed
-
- Azzam EI, de Toledo SM, Little JB. Expression of CONNEXIN43 is highly sensitive to ionizing radiation and other environmental stresses. Cancer Res. 2003;63:7128–7135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
