Stimulation of the frontal eye field reveals persistent effective connectivity after controlled behavior
- PMID: 20335465
- PMCID: PMC6634502
- DOI: 10.1523/JNEUROSCI.6198-09.2010
Stimulation of the frontal eye field reveals persistent effective connectivity after controlled behavior
Abstract
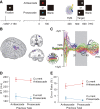
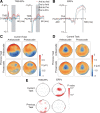
Our ability to choose nonhabitual controlled behavior instead of habitual automatic behavior is based on a flexible control mechanism subserved by neural activity representing the behavior-guiding rule. However, it has been shown that the behavior slows down more when switching from controlled to automatic behavior than vice versa. Here we show that persistent effective connectivity of the neural network after execution of controlled behavior is responsible for the behavioral slowing on a subsequent trial. We asked normal human subjects to perform a prosaccade or antisaccade task based on a cue and examined the effective connectivity of the neural network based on the pattern of neural impulse transmission induced by stimulation of the frontal eye field (FEF). Effective connectivity during the task preparation period was dependent on the task that subjects had performed on the previous trial, regardless of the upcoming task. The strength of this persistent effective connectivity was associated with saccade slowing especially on trials after controlled antisaccade. In contrast, the pattern of regional activation changed depending on the upcoming task regardless of the previous task and the decrease in activation was associated with errors in upcoming antisaccade task. These results suggest that the effective connectivity examined by FEF stimulation reflects a residual functional state of the network involved in performance of controlled antisaccade and its persistence may account for the behavioral slowing on the subsequent trial.
Figures





References
-
- Allport A, Styles EA, Hsieh SL. Shifting intentional set: exploring the dynamic control of tasks. In: Umilta C, Moscovitch M, editors. Attention and performance. Vol. XV. Cambridge, MA: MIT; 1994. pp. 421–452.
-
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. - PubMed
-
- Barton JJ, Greenzang C, Hefter R, Edelman J, Manoach DS. Switching, plasticity, and prediction in a saccadic task-switch paradigm. Exp Brain Res. 2006;168:76–87. - PubMed
-
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. - PubMed
-
- Brown MR, Vilis T, Everling S. Frontoparietal activation with preparation for antisaccades. J Neurophysiol. 2007;98:1751–1762. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
