The subthreshold relation between cortical local field potential and neuronal firing unveiled by intracellular recordings in awake rats
- PMID: 20335480
- PMCID: PMC6634481
- DOI: 10.1523/JNEUROSCI.5062-09.2010
The subthreshold relation between cortical local field potential and neuronal firing unveiled by intracellular recordings in awake rats
Abstract
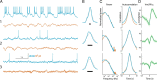
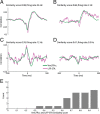
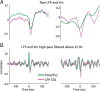
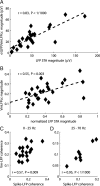
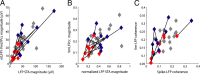
In most of the in vivo electrophysiological studies of cortical processing, which are extracellular, the spike-triggered local field potential average (LFP STA) is the measure used to estimate the correlation between the synaptic inputs of individual neuron and the local population. To understand how the magnitude and shape of LFP STA reflect the underlying correlation of synaptic activities, the membrane potential of the firing neuron has to be recorded together with the LFP. Using intracellular recordings from the cortex of awake rats, we found that for a large range of firing rates and for different behavioral states, the LFP STA represents both in its waveform and its magnitude the cross-correlation between the membrane potential of the neuron and the LFP. This data, supported by further analysis, suggests that LFP STA does not represent large network events specific to the spike times, but rather the synchrony between the mean synaptic activity of the population and the membrane potential of the single neuron, present both around spike times and in the intervals between spikes. Furthermore, it introduces a novel interpretation of the available data from unit and LFP extracellular recording experiments.
Figures




References
-
- Averbeck BB. Poisson or not Poisson: differences in spike train statistics between parietal cortical areas. Neuron. 2009;62:310–311. - PubMed
-
- Buzsaki G. Rhythms of the brain. New York: Oxford UP; 2006.
-
- Deweese MR, Zador AM. Shared and private variability in the auditory cortex. J Neurophysiol. 2004;92:1840–1855. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
