Opioid-induced long-term potentiation in the spinal cord is a presynaptic event
- PMID: 20335482
- PMCID: PMC2852319
- DOI: 10.1523/JNEUROSCI.5857-09.2010
Opioid-induced long-term potentiation in the spinal cord is a presynaptic event
Abstract
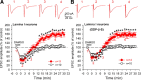
Opioids remain the mainstay of treatment for severe pain, but the associated hyperalgesia and tolerance are significant impediments to achieving adequate pain relief with opioids. Here we show that in the spinal cord, brief application of the mu-opioid receptor agonist (D-Ala(2),N-Me-Phe(4),Gly-ol(5))-enkephalin (DAMGO) at 1 mum, but not at 1-10 nm, caused an initial decrease followed by a large and long-lasting increase in the amplitude of monosynaptic EPSCs evoked from the dorsal root in approximately 50% of lamina I and II neurons. However, postsynaptic dialysis of the G-protein inhibitor had no effect on DAMGO-induced initial inhibition and long-term potentiation (LTP) in either lamina I or II neurons. DAMGO-induced LTP was associated with an increase in the paired-pulse depression ratio. Furthermore, DAMGO application and washout induced an initial decrease followed by a persistent increase in the frequency of miniature EPSCs. Bath application, but not postsynaptic dialysis, of an NMDA receptor antagonist or a calcium chelator abolished DAMGO-induced LTP. Strikingly, ablation of TRPV1-expressing primary afferents not only eliminated DAMGO-induced LTP but also prolonged DAMGO-induced inhibition of the miniature and evoked EPSCs (i.e., long-term depression). Thus, our study strongly suggests that TRPV1-expressing primary afferents play a prominent role in opioid-induced presynaptic LTP, which challenges a previous report suggesting the postsynaptic nature of this opioid-induced LTP. This excitatory effect of opioids on primary afferents can counteract the inhibitory effect of opioids on synaptic transmission at the spinal level and is likely involved in opioid-induced hyperalgesia and tolerance.
Figures






References
-
- Bashir ZI, Alford S, Davies SN, Randall AD, Collingridge GL. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature. 1991;349:156–158. - PubMed
-
- Célèrier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92:465–472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
