The spectraplakin Short stop is an actin-microtubule cross-linker that contributes to organization of the microtubule network
- PMID: 20335501
- PMCID: PMC2869377
- DOI: 10.1091/mbc.e10-01-0011
The spectraplakin Short stop is an actin-microtubule cross-linker that contributes to organization of the microtubule network
Erratum in
- Mol Biol Cell. 2010 Jun 15;21(12):2097. Zadeh, Alireza [corrected to Zadeh, Alireza Dehghani]
Abstract
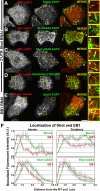
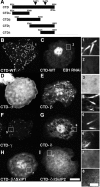
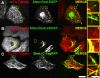
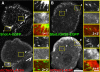
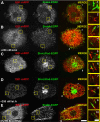
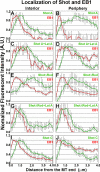
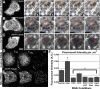
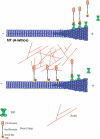
The dynamics of actin and microtubules are coordinated in a variety of cellular and morphogenetic processes; however, little is known about the molecules mediating this cytoskeletal cross-talk. We are studying Short stop (Shot), the sole Drosophila spectraplakin, as a model actin-microtubule cross-linking protein. Spectraplakins are an ancient family of giant cytoskeletal proteins that are essential for a diverse set of cellular functions; yet, we know little about the dynamics of spectraplakins and how they bridge actin filaments and microtubules. In this study we describe the intracellular dynamics of Shot and a structure-function analysis of its role as a cytoskeletal cross-linker. We find that Shot interacts with microtubules using two different mechanisms. In the cell interior, Shot binds growing plus ends through an interaction with EB1. In the cell periphery, Shot associates with the microtubule lattice via its GAS2 domain, and this pool of Shot is actively engaged as a cross-linker via its NH(2)-terminal actin-binding calponin homology domains. This cross-linking maintains microtubule organization by resisting forces that produce lateral microtubule movements in the cytoplasm. Our results provide the first description of the dynamics of these important proteins and provide key insight about how they function during cytoskeletal cross-talk.
Figures


References
-
- Bernier G., Mathieu M., De Repentigny Y., Vidal S. M., Kothary R. Cloning and characterization of mouse ACF7, a novel member of the dystonin subfamily of actin binding proteins. Genomics. 1996;38:19–29. - PubMed
-
- Drabek K., et al. Role of CLASP2 in microtubule stabilization and the regulation of persistent motility. Curr. Biol. 2006;16:2259–2264. - PubMed
-
- Fukata M., Watanabe T., Noritake J., Nakagawa M., Yamaga M., Kuroda S., Matsuura Y., Iwamatsu A., Perez F., Kaibuchi K. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–885. - PubMed
-
- Gong T. W., Besirli C. G., Lomax M. I. MACF1 gene structure: a hybrid of plectin and dystrophin. Mamm. Genome. 2001;12:852–861. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

