Inside-out regulation of L1 conformation, integrin binding, proteolysis, and concomitant cell migration
- PMID: 20335502
- PMCID: PMC2869374
- DOI: 10.1091/mbc.e09-10-0900
Inside-out regulation of L1 conformation, integrin binding, proteolysis, and concomitant cell migration
Abstract
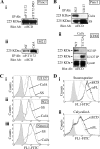
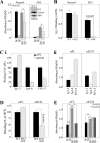
Previous reports on the expression of the cell adhesion molecule L1 in pancreatic ductal adenocarcinoma (PDAC) cells range from absent to high. Our data demonstrate that L1 is expressed in poorly differentiated PDAC cells in situ and that threonine-1172 (T1172) in the L1 cytoplasmic domain exhibits steady-state saturated phosphorylation in PDAC cells in vitro and in situ. In vitro studies support roles for casein kinase II and PKC in this modification, consistent with our prior studies using recombinant proteins. Importantly, T1172 phosphorylation drives, or is associated with, a change in the extracellular structure of L1, consistent with a potential role in regulating the shift between the closed conformation and the open, multimerized conformation of L1. We further demonstrate that these distinct conformations exhibit differential binding to integrins alphavbeta3 and alphavbeta5 and that T1172 regulates cell migration in a matrix-specific manner and is required for a disintegrin and metalloproteinase-mediated shedding of the L1 ectodomain that has been shown to regulate cell migration. These data define a specific role for T1172 of L1 in regulating aspects of pancreatic adenocarcinoma cell phenotype and suggest the need for further studies to elucidate the specific ramifications of L1 expression and T1172 phosphorylation in the pathobiology of pancreatic cancer.
Figures






Similar articles
-
Modification of the L1-CAM carboxy-terminus in pancreatic adenocarcinoma cells.Tumour Biol. 2011 Apr;32(2):347-57. doi: 10.1007/s13277-010-0127-4. Epub 2010 Nov 16. Tumour Biol. 2011. PMID: 21080252 Free PMC article.
-
Tyrosine and serine phosphorylation regulate the conformation and subsequent threonine phosphorylation of the L1 cytoplasmic domain.Biochem Biophys Res Commun. 2009 Nov 13;389(2):257-64. doi: 10.1016/j.bbrc.2009.08.143. Epub 2009 Aug 29. Biochem Biophys Res Commun. 2009. PMID: 19720049 Free PMC article.
-
Ectodomain shedding of SHPS-1 and its role in regulation of cell migration.J Biol Chem. 2004 Jul 2;279(27):27878-87. doi: 10.1074/jbc.M313085200. Epub 2004 Apr 28. J Biol Chem. 2004. PMID: 15123722
-
Role of Src kinases in the ADAM-mediated release of L1 adhesion molecule from human tumor cells.J Biol Chem. 2000 May 19;275(20):15490-7. doi: 10.1074/jbc.275.20.15490. J Biol Chem. 2000. PMID: 10809781
-
Functional Diversity of Neuronal Cell Adhesion and Recognition Molecule L1CAM through Proteolytic Cleavage.Cells. 2022 Sep 30;11(19):3085. doi: 10.3390/cells11193085. Cells. 2022. PMID: 36231047 Free PMC article. Review.
Cited by
-
Modification of the L1-CAM carboxy-terminus in pancreatic adenocarcinoma cells.Tumour Biol. 2011 Apr;32(2):347-57. doi: 10.1007/s13277-010-0127-4. Epub 2010 Nov 16. Tumour Biol. 2011. PMID: 21080252 Free PMC article.
-
T cell receptor signaling can directly enhance the avidity of CD28 ligand binding.PLoS One. 2014 Feb 24;9(2):e89263. doi: 10.1371/journal.pone.0089263. eCollection 2014. PLoS One. 2014. PMID: 24586641 Free PMC article.
-
L1cam-mediated developmental processes of the nervous system are differentially regulated by proteolytic processing.Sci Rep. 2019 Mar 6;9(1):3716. doi: 10.1038/s41598-019-39884-x. Sci Rep. 2019. PMID: 30842511 Free PMC article.
-
Mitogen-activated protein kinase modulates ethanol inhibition of cell adhesion mediated by the L1 neural cell adhesion molecule.Proc Natl Acad Sci U S A. 2013 Apr 2;110(14):5683-8. doi: 10.1073/pnas.1221386110. Epub 2013 Feb 19. Proc Natl Acad Sci U S A. 2013. PMID: 23431142 Free PMC article.
-
Different Shades of L1CAM in the Pathophysiology of Cancer Stem Cells.J Clin Med. 2020 May 16;9(5):1502. doi: 10.3390/jcm9051502. J Clin Med. 2020. PMID: 32429448 Free PMC article. Review.
References
-
- Bardeesy N., DePinho R. A. Pancreatic cancer biology and genetics. Nat. Rev. 2002;2:897–909. - PubMed
-
- Beer S., Oleszewski M., Gutwein P., Geiger C., Altevogt P. Metalloproteinase-mediated release of the ectodomain of L1 adhesion molecule. J. Cell Sci. 1999;112:2667–2675. - PubMed
-
- Bouvet M., Gamagami R. A., Gilpin E. A., Romeo O., Sasson A., Easter D., Moossa A. R. Factors influencing survival after resection for periampullary neoplasms. Am. J. Surg. 2000;180:13–17. - PubMed
-
- Burden-Gulley S. M., Pendergast M., Lemmon V. The role of cell adhesion molecule L1 in axonal extension, growth cone motility, and signal transduction. Cell Tissue Res. 1997;290:415–422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

