Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney
- PMID: 20335657
- PMCID: PMC2846062
- DOI: 10.1172/JCI41376
Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney
Abstract
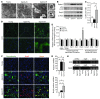
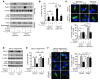
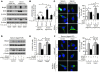
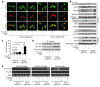
Mitochondrial oxidative damage is a basic mechanism of aging, and multiple studies demonstrate that this process is attenuated by calorie restriction (CR). However, the molecular mechanism that underlies the beneficial effect of CR on mitochondrial dysfunction is unclear. Here, we investigated in mice the mechanisms underlying CR-mediated protection against hypoxia in aged kidney, with a special focus on the role of the NAD-dependent deacetylase sirtuin 1 (Sirt1), which is linked to CR-related longevity in model organisms, on mitochondrial autophagy. Adult-onset and long-term CR in mice promoted increased Sirt1 expression in aged kidney and attenuated hypoxia-associated mitochondrial and renal damage by enhancing BCL2/adenovirus E1B 19-kDa interacting protein 3-dependent (Bnip3-dependent) autophagy. Culture of primary renal proximal tubular cells (PTCs) in serum from CR mice promoted Sirt1-mediated forkhead box O3 (Foxo3) deacetylation. This activity was essential for expression of Bnip3 and p27Kip1 and for subsequent autophagy and cell survival of PTCs under hypoxia. Furthermore, the kidneys of aged Sirt1+/- mice were resistant to CR-mediated improvement in the accumulation of damaged mitochondria under hypoxia. These data highlight the role of the Sirt1-Foxo3 axis in cellular adaptation to hypoxia, delineate a molecular mechanism of the CR-mediated antiaging effect, and could potentially direct the design of new therapies for age- and hypoxia-related tissue damage.
Figures




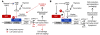
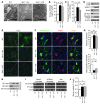
Comment in
-
The role of Sirt1 in renal rejuvenation and resistance to stress.J Clin Invest. 2010 Apr;120(4):1026-8. doi: 10.1172/JCI42184. Epub 2010 Mar 24. J Clin Invest. 2010. PMID: 20335654 Free PMC article.
Similar articles
-
The role of Sirt1 in renal rejuvenation and resistance to stress.J Clin Invest. 2010 Apr;120(4):1026-8. doi: 10.1172/JCI42184. Epub 2010 Mar 24. J Clin Invest. 2010. PMID: 20335654 Free PMC article.
-
Polydatin: a potential NAFLD therapeutic drug that regulates mitochondrial autophagy through SIRT3-FOXO3-BNIP3 and PINK1-PRKN mechanisms - a network pharmacology and experimental investigation.Chem Biol Interact. 2024 Aug 1;398:111110. doi: 10.1016/j.cbi.2024.111110. Epub 2024 Jun 13. Chem Biol Interact. 2024. PMID: 38876248
-
Short-term calorie restriction protects against renal senescence of aged rats by increasing autophagic activity and reducing oxidative damage.Mech Ageing Dev. 2013 Nov-Dec;134(11-12):570-9. doi: 10.1016/j.mad.2013.11.006. Epub 2013 Nov 27. Mech Ageing Dev. 2013. PMID: 24291536
-
The Role of Calorie Restriction in Modifying the Ageing Process through the Regulation of SIRT1 Expression.Subcell Biochem. 2024;107:173-181. doi: 10.1007/978-3-031-66768-8_8. Subcell Biochem. 2024. PMID: 39693024 Review.
-
Redox regulation of SIRT1 in inflammation and cellular senescence.Free Radic Biol Med. 2013 Aug;61:95-110. doi: 10.1016/j.freeradbiomed.2013.03.015. Epub 2013 Mar 27. Free Radic Biol Med. 2013. PMID: 23542362 Free PMC article. Review.
Cited by
-
SIRT1 and Kidney Function.Kidney Dis (Basel). 2016 Mar;1(4):258-65. doi: 10.1159/000440967. Epub 2015 Oct 13. Kidney Dis (Basel). 2016. PMID: 27536685 Free PMC article. Review.
-
Autophagy in kidney health and disease.Antioxid Redox Signal. 2014 Jan 20;20(3):519-37. doi: 10.1089/ars.2013.5363. Epub 2013 Jun 28. Antioxid Redox Signal. 2014. PMID: 23642034 Free PMC article. Review.
-
Autophagy is involved in aldosterone‑induced mesangial cell proliferation.Mol Med Rep. 2016 Nov;14(5):4638-4642. doi: 10.3892/mmr.2016.5807. Epub 2016 Oct 5. Mol Med Rep. 2016. PMID: 27748808 Free PMC article.
-
Aldosterone induces p21-regulated apoptosis via increased synthesis and secretion of tumour necrosis factor-α in human proximal tubular cells.Clin Exp Pharmacol Physiol. 2012 Oct;39(10):858-63. doi: 10.1111/1440-1681.12001. Clin Exp Pharmacol Physiol. 2012. PMID: 23013131 Free PMC article.
-
Role of SIRT1 in HIV-associated kidney disease.Am J Physiol Renal Physiol. 2020 Aug 1;319(2):F335-F344. doi: 10.1152/ajprenal.00140.2020. Epub 2020 Jul 13. Am J Physiol Renal Physiol. 2020. PMID: 32657157 Free PMC article.
References
-
- Baylis C, Corman B. The aging kidney: insights from experimental studies. J Am Soc Nephrol. 1998;9(4):699–709. - PubMed
-
- Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41(1):1–12. - PubMed
-
- Epstein M. Aging and the kidney. J Am Soc Nephrol. 1996;7(8):1106–1122. - PubMed
-
- Cutler RG. Human longevity and aging: possible role of reactive oxygen species. Ann N Y Acad Sci. 1991;621:1–28. - PubMed
-
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82(1):47–95. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

