Mast cell chymase limits the cardiac efficacy of Ang I-converting enzyme inhibitor therapy in rodents
- PMID: 20335663
- PMCID: PMC2846039
- DOI: 10.1172/JCI39345
Mast cell chymase limits the cardiac efficacy of Ang I-converting enzyme inhibitor therapy in rodents
Abstract
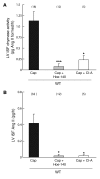
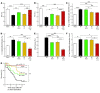
Ang I-converting enzyme (ACE) inhibitors are widely believed to suppress the deleterious cardiac effects of Ang II by inhibiting locally generated Ang II. However, the recent demonstration that chymase, an Ang II-forming enzyme stored in mast cell granules, is present in the heart has added uncertainty to this view. As discussed here, using microdialysis probes tethered to the heart of conscious mice, we have shown that chronic ACE inhibitor treatment did not suppress Ang II levels in the LV interstitial fluid (ISF) despite marked inhibition of ACE. However, chronic ACE inhibition caused a marked bradykinin/B2 receptor-mediated increase in LV ISF chymase activity that was not observed in mast cell-deficient KitW/KitW-v mice. In chronic ACE inhibitor-treated mast cell-sufficient littermates, chymase inhibition decreased LV ISF Ang II levels substantially, indicating the importance of mast cell chymase in regulating cardiac Ang II levels. Chymase-dependent processing of other regulatory peptides also promotes inflammation and tissue remodeling. We found that combined chymase and ACE inhibition, relative to ACE inhibition alone, improved LV function, decreased adverse cardiac remodeling, and improved survival after myocardial infarction in hamsters. These results suggest that chymase inhibitors could be a useful addition to ACE inhibitor therapy in the treatment of heart failure.
Figures






Comment in
-
The one-two punch: knocking out angiotensin II in the heart.J Clin Invest. 2010 Apr;120(4):1028-31. doi: 10.1172/JCI42644. Epub 2010 Mar 24. J Clin Invest. 2010. PMID: 20335650 Free PMC article.
Similar articles
-
Involvement of chymase-mediated angiotensin II generation in blood pressure regulation.J Clin Invest. 2004 Jul;114(1):112-20. doi: 10.1172/JCI20805. J Clin Invest. 2004. PMID: 15232618 Free PMC article.
-
Cellular localization and regional distribution of an angiotensin II-forming chymase in the heart.J Clin Invest. 1993 Apr;91(4):1269-81. doi: 10.1172/JCI116325. J Clin Invest. 1993. PMID: 7682566 Free PMC article.
-
Contributions of ACE and mast cell chymase to endogenous angiotensin II generation and leucocyte recruitment in vivo.Cardiovasc Res. 2011 Oct 1;92(1):48-56. doi: 10.1093/cvr/cvr147. Epub 2011 May 27. Cardiovasc Res. 2011. PMID: 21622682
-
[Angiotensin II formation by chymase in the cardiovascular tissue].Nihon Yakurigaku Zasshi. 1998 Sep;112(3):203-12. doi: 10.1254/fpj.112.203. Nihon Yakurigaku Zasshi. 1998. PMID: 9793075 Review. Japanese.
-
Pathological roles of angiotensin II produced by mast cell chymase and the effects of chymase inhibition in animal models.Pharmacol Ther. 2006 Dec;112(3):668-76. doi: 10.1016/j.pharmthera.2006.05.008. Epub 2006 Jul 11. Pharmacol Ther. 2006. PMID: 16837049 Review.
Cited by
-
RAS-Mediated Adaptive Mechanisms in Cardiovascular Tissues: Confounding Factors of RAS Blockade Therapy and Alternative Approaches.Cardiorenal Med. 2012 Dec;2(4):268-280. doi: 10.1159/000343456. Epub 2012 Oct 27. Cardiorenal Med. 2012. PMID: 23381810 Free PMC article.
-
Kinin B2 Receptor Mediates Cisplatin-Induced Painful Peripheral Neuropathy by Intracellular Kinase Pathways and TRPA1 Channel Sensitisation.Pharmaceuticals (Basel). 2023 Jul 4;16(7):959. doi: 10.3390/ph16070959. Pharmaceuticals (Basel). 2023. PMID: 37513871 Free PMC article.
-
Myofilament dysfunction as an emerging mechanism of volume overload heart failure.Pflugers Arch. 2014 Jun;466(6):1065-77. doi: 10.1007/s00424-014-1455-9. Epub 2014 Feb 1. Pflugers Arch. 2014. PMID: 24488008 Review.
-
Mast Cells in Cardiac Fibrosis: New Insights Suggest Opportunities for Intervention.Front Immunol. 2019 Mar 28;10:580. doi: 10.3389/fimmu.2019.00580. eCollection 2019. Front Immunol. 2019. PMID: 31001246 Free PMC article. Review.
-
Critical role of the chymase/angiotensin-(1-12) axis in modulating cardiomyocyte contractility.Int J Cardiol. 2018 Aug 1;264:137-144. doi: 10.1016/j.ijcard.2018.03.066. Epub 2018 Apr 21. Int J Cardiol. 2018. PMID: 29685688 Free PMC article.
References
-
- Corvol P, Eyries M, and Soubrier F. Peptidyl-dipeptidase A/angiotensin I-converting enzyme. In: Barrett AJ, Rawlings ND, Woessner JF, eds.Handbook of Proteolytic Enzymes. 2nd ed. New York, NY: Academic Press, Inc. 2004:332–346.
-
- N Engl J Med. 1992;327(10):685–691. - PubMed
-
- Pfeffer MA. Left ventricular remodeling after acute myocardial infarction. Annu Rev Med. 1995;46:455–466. - PubMed
-
- Borghi C, Marino P, Zardini P, Magnani B, Collatina S, Ambrosioni E. Post acute myocardial infarction: the Fosinopril in Acute Myocardial Infarction Study (FAMIS). Am J Hypertens. 1997;10(10 Pt 2):247S–254S. - PubMed
-
- Urata H, Healy B, Stewart RW, Bumpus FM, Husain A. Angiotensin II-forming pathways in normal and failing human hearts. Circ Res. 1990;66(4):883–890. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

