Seeking polymeric prodrugs of norfloxacin. Part 2. Synthesis and structural analysis of polyurethane conjugates
- PMID: 20335951
- PMCID: PMC6263196
- DOI: 10.3390/molecules15020842
Seeking polymeric prodrugs of norfloxacin. Part 2. Synthesis and structural analysis of polyurethane conjugates
Abstract
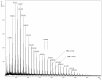
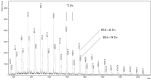
Oligo(epsilon-caprolactone) and oligolactide were synthesized via ring-opening polymerization of cyclic esters in the presence of creatinine as initiators. Thus obtained oligomers were successfully used in the synthesis of novel polyurethane conjugates of norfloxacin. The structures of the polymers and conjugates were elucidated by means of MALDI-TOF MS, NMR and IR studies.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

