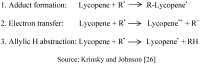Revealing the power of the natural red pigment lycopene
- PMID: 20335956
- PMCID: PMC6263198
- DOI: 10.3390/molecules15020959
Revealing the power of the natural red pigment lycopene
Abstract
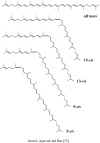
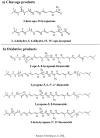
By-products derived from food processing are attractive source for their valuable bioactive components and color pigments. These by-products are useful for development as functional foods, nutraceuticals, food ingredients, additives, and also as cosmetic products. Lycopene is a bioactive red colored pigment naturally occurring in plants. Industrial by-products obtained from the plants are the good sources of lycopene. Interest in lycopene is increasing due to increasing evidence proving its preventive properties toward numerous diseases. In vitro, in vivo and ex vivo studies have demonstrated that lycopene-rich foods are inversely associated to diseases such as cancers, cardiovascular diseases, diabetes, and others. This paper also reviews the properties, absorption, transportation, and distribution of lycopene and its by-products in human body. The mechanism of action and interaction of lycopene with other bioactive compounds are also discussed, because these are the crucial features for beneficial role of lycopene. However, information on the effect of food processing on lycopene stability and availability was discussed for better understanding of its characteristics.
Figures
References
-
- Mortensen A. Carotenoids and other pigments as natural colorants. Pure Appl. Chem. 2006;78:1477–1491. doi: 10.1351/pac200678081477. - DOI
-
- Rodriguez-Amaya D.B., Kimura M. Harvestplus Handbook for Carotenoid Analysis. IFPRI and CIAT; Washington, DC, USA: 2004. Carotenoids in foods; pp. 2–7.
-
- Rodriguez-Amaya D.B. A Guide to Carotenoid Analysis in Foods. ILSI Press; Washington, D.C., USA: 2001. pp. 1–45.
-
- Focus on Pigments. World spends more than $50 M on lycopene red. Focus Pigm. 2007;4:3–4.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources