Isolation and identification of two new polyynes from a North American ethnic medicinal plant--Oplopanax horridus (Smith) Miq
- PMID: 20335964
- PMCID: PMC6263199
- DOI: 10.3390/molecules15021089
Isolation and identification of two new polyynes from a North American ethnic medicinal plant--Oplopanax horridus (Smith) Miq
Abstract
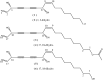
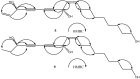
Two new polyynes, named oplopantriol A (5) and oplopantriol B (6), were isolated from the root bark of Oplopanax horridus (Smith) Miq, an ethnic medicinal plant of North America, along with four known polyynes: (3S,8S)-falcarindiol (1), oplopandiol (2), (11S,16S,9Z)-9,17-octadecadiene-12,14-diyne-1,11,16-triol, 1-acetate (3) and oplopandiol acetate (4). The structures of the new compounds were elucidated by detailed spectroscopic analyses, including 1D and 2D NMR techniques and chemical methods. The absolute configurations of the new compounds 5 and 6 were determined by comparing their optical rotation values with the hydrolysis products of the known compounds 3 and 4, respectively, derived from the same plant. On the basis of an analysis of their physical and chemical properties we show that the alkaline hydrolysis of 3 and 4 afforded the new compounds 5 and 6, respectively.
Figures
References
-
- Wu C.Y. Flora of China. Vol. 54. Science Press; Beijing, China: 1988. p. 16.
-
- Takeda K., Minato H., Ishikawa M. Studies on sesquiterpenoids—XII Structure and absolute configuration of oplopanone, a new sesquiterpene from oplopanax japonicus (NAKAI) NAKAI. Tetrahedron. 1966;22(Suppl. 7):219–225. doi: 10.1016/S0040-4020(01)99108-2. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources