Prodrug approach for increasing cellular glutathione levels
- PMID: 20335977
- PMCID: PMC6257297
- DOI: 10.3390/molecules15031242
Prodrug approach for increasing cellular glutathione levels
Abstract
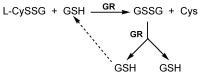
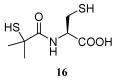
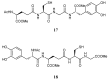
Reduced glutathione (GSH) is the most abundant non-protein thiol in mammalian cells and the preferred substrate for several enzymes in xenobiotic metabolism and antioxidant defense. It plays an important role in many cellular processes, such as cell differentiation, proliferation and apoptosis. GSH deficiency has been observed in aging and in a wide range of pathologies, including neurodegenerative disorders and cystic fibrosis (CF), as well as in several viral infections. Use of GSH as a therapeutic agent is limited because of its unfavorable biochemical and pharmacokinetic properties. Several reports have provided evidence for the use of GSH prodrugs able to replenish intracellular GSH levels. This review discusses different strategies for increasing GSH levels by supplying reversible bioconjugates able to cross the cellular membrane more easily than GSH and to provide a source of thiols for GSH synthesis.
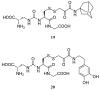
Figures






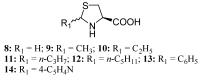


References
-
- Meister A. Glutathione metabolism and its selective modification. J. Biol. Chem. 1988;263:17205–17208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

