Stereodynamic investigation of labile stereogenic centres in dihydroartemisinin
- PMID: 20335983
- PMCID: PMC6257325
- DOI: 10.3390/molecules15031309
Stereodynamic investigation of labile stereogenic centres in dihydroartemisinin
Abstract
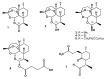
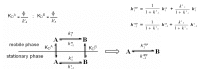
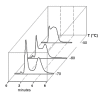
Since its identification in the early 1970s, artemisinin, as well as semi-synthetic derivatives and synthetic trioxanes, have been used in malaria therapy. Reduction of artemisinin by NaBH4 produced dihydroartemisinin (DHA), and yielded a new stereochemically labile centre at C-10, which, in turn, provided two interconverting lactol hemiacetal epimers (namely alpha and beta), whose rate of interconversion depends on buffer, pH, and solvent polarity. Since interconversion of the two epimers occurred on a chromatographic time-scale, this prompted a thorough investigation of the phenomenon as a crucial requisite of any analytical method aimed at quantitating this family of drugs. In this critical review we discuss the current importance of the on-column epimerization of DHA in the development of analytical methods aimed at quantifying the drug, with the purpose of identifying the optimal conditions to minimize on-column epimerization while achieving the best selectivity and efficiency of the overall separation.
Figures





References
-
- Guidelines for the Treatment of Malaria. World Health Organization; Geneva, Switzerland: 2006.
-
- Klayman D.L. Qinghaosu (artemisinin): An Anti-malarial Drug from China. Science. 1985;228:1049–1055. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

