Synthesis of lysophospholipids
- PMID: 20335986
- PMCID: PMC6257299
- DOI: 10.3390/molecules15031354
Synthesis of lysophospholipids
Abstract
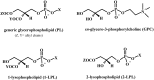
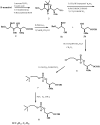
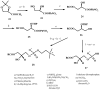
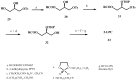
New synthetic methods for the preparation of biologically active phospholipids and lysophospholipids (LPLs) are very important in solving problems of membrane-chemistry and biochemistry. Traditionally considered just as second-messenger molecules regulating intracellular signalling pathways, LPLs have recently shown to be involved in many physiological and pathological processes such as inflammation, reproduction, angiogenesis, tumorogenesis, atherosclerosis and nervous system regulation. Elucidation of the mechanistic details involved in the enzymological, cell-biological and membrane-biophysical roles of LPLs relies obviously on the availability of structurally diverse compounds. A variety of chemical and enzymatic routes have been reported in the literature for the synthesis of LPLs: the enzymatic transformation of natural glycerophospholipids (GPLs) using regiospecific enzymes such as phospholipases A1 (PLA1), A2 (PLA2) phospholipase D (PLD) and different lipases, the coupling of enzymatic processes with chemical transformations, the complete chemical synthesis of LPLs starting from glycerol or derivatives. In this review, chemo-enzymatic procedures leading to 1- and 2-LPLs will be described.
Figures
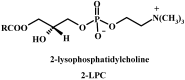
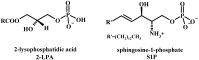




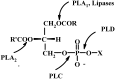

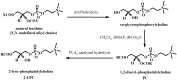
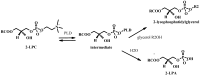
References
-
- Vance D.E., Vance J.E. Biochemistry of Lipids, Lipoproteins and membranes. 4th ed. Elsevier Science; Amsterdam, The Netherlands: 2002. pp. 315–340.
-
- Dowhan W. The role of phospholipids in cell function. Adv. Lipobiol. 1997;2:79–107. doi: 10.1016/S1874-5245(97)80006-7. - DOI
-
- Barenholz Y., Cevc G. Structure and Properties of Membranes. In: Baskin A., Norde W., editors. Physical Chemistry of Biological Interfaces. Marcel Dekker; New York, NY, USA: 2000. pp. 171–242.
-
- Goetzl E.J., An S. Diversity of cellular receptors and functions for the lysophospholipid growth factors lysophosphatidic acid and sphingosine 1-phosphate. FASEB J. 1998;12:1589–1598. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

