A simple and rapid ESI-LC-MS/MS method for simultaneous screening of doping agents in urine samples
- PMID: 20336223
- PMCID: PMC2841238
- DOI: 10.4103/0253-7613.51347
A simple and rapid ESI-LC-MS/MS method for simultaneous screening of doping agents in urine samples
Abstract
Objective: The use of performance enhancing substances is banned in sports by the World Anti-Doping Agency (WADA). Though most prohibited substances can be detected by GC/MS, inclusion of corticosteroids and designer drugs has made it essential to detect these critical doping agents on LC/MS/MS due to their better separation and detection.
Materials and methods: A common extraction procedure for the isolation of acidic, basic and neutral drugs from urine samples was developed. A total of 28 doping drugs were analyzed on API 3200 Triple quadrupole mass spectrometer using C18 column in atmospheric pressure electrospray ionization. The mobile phase composition was a mixture of 1% formic acid and acetonitrile with gradient time period.
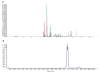
Results: The method developed was very sensitive for detection of 28 doping agents. The linearity was performed for each drug and the total recovery percentage ranged from 57 to 114. Limit of detection is found to be 0.5 ng/ml for carboxy finasteride and 1-5 ng/ml for other drugs. The method was successfully used to detect positive urine samples of 3-OH-stanozolol, methyl phenidate, mesocarb, clomiphene metabolite and carboxy finasteride.
Conclusion: The method developed based on controlled pH extraction method and HPLC-mass spectrometry analysis allowed better identification and confirmation of glucocorticosteroids and a few other drugs in different categories. The validated method has been used successfully for testing of 1000 In-competition samples. The method helped in detection of chemically and pharmacologically different banned drugs in urine in a single short run at a minimum required performance limit set by WADA.
Keywords: Detection limit; LC/MS/MS; glucocorticosteroids; validation.
Figures

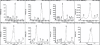


Similar articles
-
Ultra high performance liquid chromatography tandem mass spectrometry determination and profiling of prohibited steroids in human biological matrices. A review.J Chromatogr B Analyt Technol Biomed Life Sci. 2013 May 15;927:22-36. doi: 10.1016/j.jchromb.2012.12.003. Epub 2012 Dec 20. J Chromatogr B Analyt Technol Biomed Life Sci. 2013. PMID: 23317577 Review.
-
A novel study of screening and confirmation of modafinil, adrafinil and their metabolite modafinilic acid under EI-GC-MS and ESI-LC-MS-MS ionization.Indian J Pharmacol. 2009 Dec;41(6):278-83. doi: 10.4103/0253-7613.59928. Indian J Pharmacol. 2009. PMID: 20407560 Free PMC article.
-
A rapid screening LC-MS/MS method based on conventional HPLC pumps for the analysis of low molecular weight xenobiotics: application to doping control analysis.Drug Test Anal. 2010 Jul;2(7):311-22. doi: 10.1002/dta.148. Drug Test Anal. 2010. PMID: 20818799
-
Sensitive determination of prohibited drugs in dried blood spots (DBS) for doping controls by means of a benchtop quadrupole/Orbitrap mass spectrometer.Anal Bioanal Chem. 2012 May;403(5):1279-89. doi: 10.1007/s00216-011-5655-2. Epub 2012 Jan 10. Anal Bioanal Chem. 2012. PMID: 22231507
-
Forced degradation and impurity profiling: recent trends in analytical perspectives.J Pharm Biomed Anal. 2013 Dec;86:11-35. doi: 10.1016/j.jpba.2013.07.013. Epub 2013 Jul 31. J Pharm Biomed Anal. 2013. PMID: 23969330 Review.
Cited by
-
A preliminary study on urinary excretion patterns of methylprednisolone after oral and intra-articular administration and effect on endogenous glucocorticosteroids profile.Indian J Pharmacol. 2021 Nov-Dec;53(6):480-483. doi: 10.4103/ijp.ijp_946_20. Indian J Pharmacol. 2021. PMID: 34975136 Free PMC article.
-
Application of LC-MS/MS for quantitative analysis of glucocorticoids and stimulants in biological fluids.J Pharm Anal. 2013 Oct;3(5):341-348. doi: 10.1016/j.jpha.2013.03.005. Epub 2013 Apr 2. J Pharm Anal. 2013. PMID: 29403837 Free PMC article.
-
Generic sample preparation combined with high-resolution liquid chromatography-time-of-flight mass spectrometry for unification of urine screening in doping-control laboratories.Anal Bioanal Chem. 2010 Apr;396(7):2583-98. doi: 10.1007/s00216-010-3484-3. Epub 2010 Feb 16. Anal Bioanal Chem. 2010. PMID: 20155493 Free PMC article.
-
Novel dummy molecularly imprinted polymer for simultaneous solid-phase extraction of stanozolol metabolites from urine.Anal Bioanal Chem. 2024 Jun;416(14):3335-3347. doi: 10.1007/s00216-024-05285-x. Epub 2024 Apr 25. Anal Bioanal Chem. 2024. PMID: 38661944 Free PMC article.
References
-
- Larry Bowers D. Analytical advances in detection of performance enhancing compounds. Clin Chem. 1997;43(7):1299–304. - PubMed
-
- Duntas LH, Parisis C. Doping: A challenge to the endocrinologist: A reappraisal in view of the Olympic games of 2004. Hormones. 2003;2:35–42. - PubMed
-
- Reddy MI, Beotra A, Jain S, Kaur T, Lal R. Purification of urine samples to improve detection limit of anabolic agents. Indian J Pharmacol. 2007;39:39–41.
-
- Thevis M, Schanzer W. Mass spectrometry in doping control analysis. Curr Organ Chem. 2005;9:825–48.
-
- Fouracre C, Kazlauskas R. Diuretics screening and confirmation using LC/MS/MS. Recent advances in doping analysis (9) Sport und Buch Strauβ, Koln. 2001. pp. 321–6.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

