Monophosphoryl lipid A plus IFNgamma maturation of dendritic cells induces antigen-specific CD8+ cytotoxic T cells with high cytolytic potential
- PMID: 20336295
- PMCID: PMC11030766
- DOI: 10.1007/s00262-010-0843-z
Monophosphoryl lipid A plus IFNgamma maturation of dendritic cells induces antigen-specific CD8+ cytotoxic T cells with high cytolytic potential
Abstract
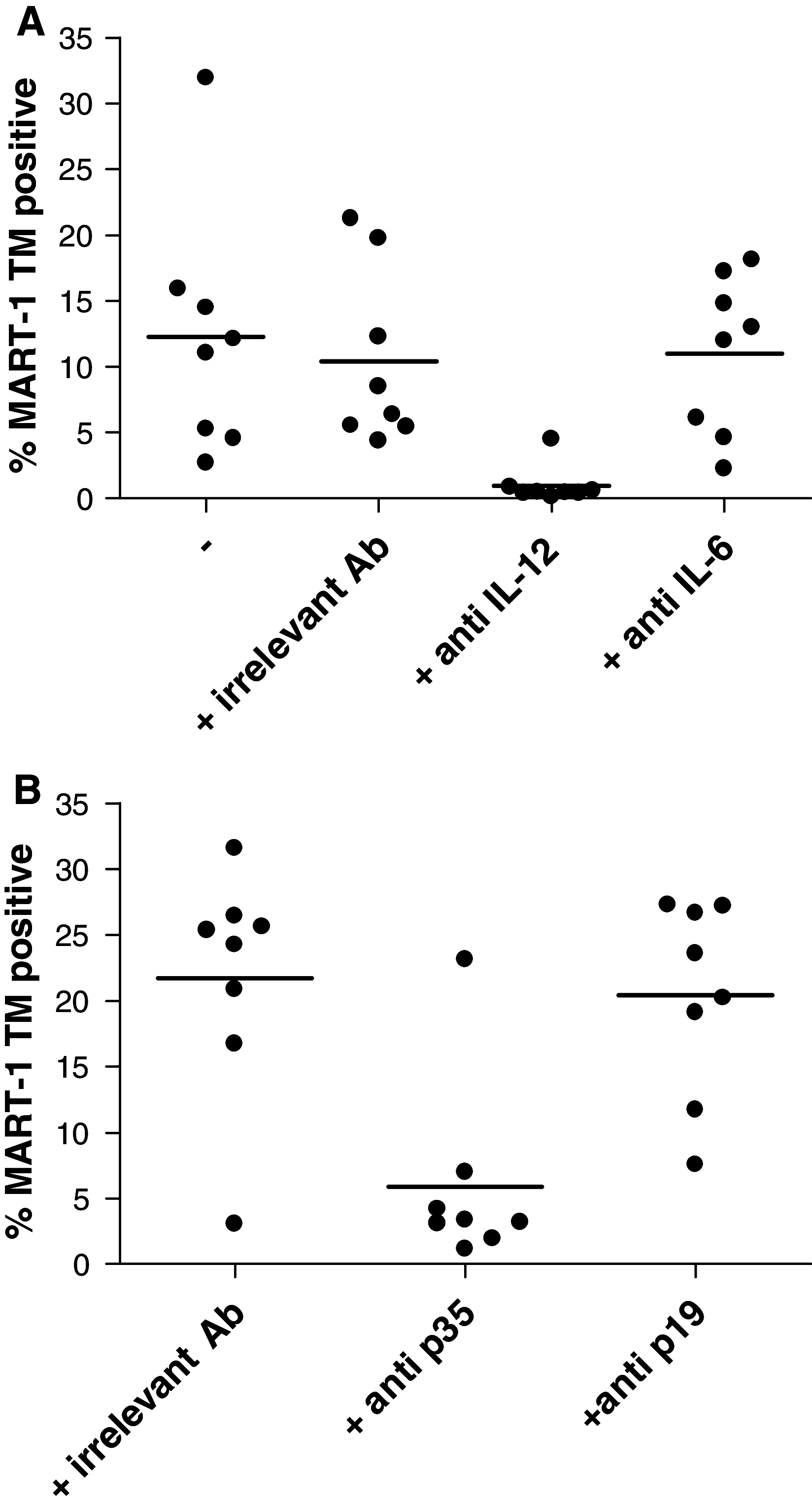
Dendritic cells (DCs) are promising antigen presenting cells for cancer treatment. Previously, we showed that the combination of monophosphoryl lipid A (MPLA) with IFNgamma generates mature DCs that produce IL-12 and polarize CD4(+) T cells towards a Th1 phenotype. Here, we extended these observations by showing that the DCs generated with the clinical grade maturation cocktail of MPLA/IFNgamma induce superior tumour antigen-specific CD8(+) CTL responses compared to the cytokine cocktail matured DCs that are currently used in the clinic. MPLA/IFNgamma DCs can induce CTL responses in healthy individuals as well as in melanoma patients. The CTL induction was mainly dependent on the IL-12 produced by the MPLA/IFNgamma DCs. The high amounts of induced CTLs are functional as they produce IFNgamma and lyse target cells and this cytolytic activity is antigen specific and HLA restricted. Furthermore, the CTLs proved to kill tumour cells expressing endogenous tumour antigen in vitro. Therefore, MPLA/IFNgamma DCs are very promising for the use in future cancer immunotherapy.
Figures



Similar articles
-
The clinical grade maturation cocktail monophosphoryl lipid A plus IFNgamma generates monocyte-derived dendritic cells with the capacity to migrate and induce Th1 polarization.Vaccine. 2007 Oct 10;25(41):7145-52. doi: 10.1016/j.vaccine.2007.07.031. Epub 2007 Aug 6. Vaccine. 2007. PMID: 17719152
-
An accelerated, clinical-grade protocol to generate high yields of type 1-polarizing messenger RNA-loaded dendritic cells for cancer vaccination.Cytotherapy. 2018 Sep;20(9):1164-1181. doi: 10.1016/j.jcyt.2018.06.006. Epub 2018 Aug 16. Cytotherapy. 2018. PMID: 30122654
-
Helper activity of natural killer cells during the dendritic cell-mediated induction of melanoma-specific cytotoxic T cells.J Immunother. 2011 Apr;34(3):270-8. doi: 10.1097/CJI.0b013e31820b370b. J Immunother. 2011. PMID: 21389871 Free PMC article.
-
Third generation dendritic cell vaccines for tumor immunotherapy.Eur J Cell Biol. 2012 Jan;91(1):53-8. doi: 10.1016/j.ejcb.2011.01.012. Epub 2011 Mar 25. Eur J Cell Biol. 2012. PMID: 21439674 Review.
-
Toll-like receptor agonists: are they good adjuvants?Cancer J. 2010 Jul-Aug;16(4):382-91. doi: 10.1097/PPO.0b013e3181eaca65. Cancer J. 2010. PMID: 20693851 Free PMC article. Review.
Cited by
-
Prostaglandin E2 in a TLR3- and 7/8-agonist-based DC maturation cocktail generates mature, cytokine-producing, migratory DCs but impairs antigen cross-presentation to CD8+ T cells.Cancer Immunol Immunother. 2020 Jun;69(6):1029-1042. doi: 10.1007/s00262-019-02470-1. Epub 2020 Feb 25. Cancer Immunol Immunother. 2020. PMID: 32100075 Free PMC article.
-
Sweeten PAMPs: Role of Sugar Complexed PAMPs in Innate Immunity and Vaccine Biology.Front Immunol. 2013 Sep 2;4:248. doi: 10.3389/fimmu.2013.00248. Front Immunol. 2013. PMID: 24032031 Free PMC article. Review.
-
Monophosphoryl lipid A-induced pro-inflammatory cytokine expression does not require CD14 in primary human dendritic cells.Inflamm Res. 2016 Jun;65(6):449-58. doi: 10.1007/s00011-016-0927-0. Epub 2016 Mar 18. Inflamm Res. 2016. PMID: 26994069
-
Primed tumor-reactive multifunctional CD62L+ human CD8+ T cells for immunotherapy.Cancer Immunol Immunother. 2011 Feb;60(2):173-86. doi: 10.1007/s00262-010-0928-8. Epub 2010 Oct 24. Cancer Immunol Immunother. 2011. PMID: 20972785 Free PMC article.
-
Activating a collaborative innate-adaptive immune response to control metastasis.Cancer Cell. 2021 Oct 11;39(10):1361-1374.e9. doi: 10.1016/j.ccell.2021.08.005. Epub 2021 Sep 2. Cancer Cell. 2021. PMID: 34478639 Free PMC article.
References
-
- Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

