Improved management of lysosomal glucosylceramide levels in a mouse model of type 1 Gaucher disease using enzyme and substrate reduction therapy
- PMID: 20336375
- PMCID: PMC3683842
- DOI: 10.1007/s10545-010-9072-z
Improved management of lysosomal glucosylceramide levels in a mouse model of type 1 Gaucher disease using enzyme and substrate reduction therapy
Abstract
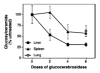
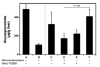
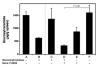
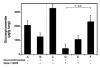
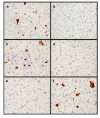
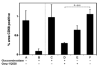
Gaucher disease is caused by a deficiency of the lysosomal enzyme glucocerebrosidase (acid beta-glucosidase), with consequent cellular accumulation of glucosylceramide (GL-1). The disease is managed by intravenous administrations of recombinant glucocerebrosidase (imiglucerase), although symptomatic patients with mild to moderate type 1 Gaucher disease for whom enzyme replacement therapy (ERT) is not an option may also be treated by substrate reduction therapy (SRT) with miglustat. To determine whether the sequential use of both ERT and SRT may provide additional benefits, we compared the relative pharmacodynamic efficacies of separate and sequential therapies in a murine model of Gaucher disease (D409V/null). As expected, ERT with recombinant glucocerebrosidase was effective in reducing the burden of GL-1 storage in the liver, spleen, and lung of 3-month-old Gaucher mice. SRT using a novel inhibitor of glucosylceramide synthase (Genz-112638) was also effective, albeit to a lesser degree than ERT. Animals administered recombinant glucocerebrosidase and then Genz-112638 showed the lowest levels of GL-1 in all the visceral organs and a reduced number of Gaucher cells in the liver. This was likely because the additional deployment of SRT following enzyme therapy slowed the rate of reaccumulation of GL-1 in the affected organs. Hence, in patients whose disease has been stabilized by intravenously administered recombinant glucocerebrosidase, orally administered SRT with Genz-112638 could potentially be used as a convenient maintenance therapy. In patients naïve to treatment, ERT followed by SRT could potentially accelerate clearance of the offending substrate.
Figures
References
-
- Beutler E, Grabowski GA. Gaucher disease. In: Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular basis of inherited disease (Scriver CR) McGraw-Hill; New York: 2001. pp. 3635–3668.
-
- Charrow J, Andersson HC, Kaplan P, et al. Enzyme replacement therapy and monitoring for children with type 1 Gaucher disease: consensus recommendations. J Pediatr. 2004;144:112–120. - PubMed
-
- Cox TM, Aerts JM, Andria G, et al. The role of the iminosugar N-butyldeoxynojirimycin (miglustat) in the management of type 1 (non-neuropathic) Gaucher disease: a position statement. J Inherit Metab Dis. 2003;26:513–526. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

