A phase synchrony measure for quantifying dynamic functional integration in the brain
- PMID: 20336687
- PMCID: PMC6870375
- DOI: 10.1002/hbm.21000
A phase synchrony measure for quantifying dynamic functional integration in the brain
Abstract
The temporal coordination of neural activity within structural networks of the brain has been posited as a basis for cognition. Changes in the frequency and similarity of oscillating electrical potentials emitted by neuronal populations may reflect the means by which networks of the brain carry out functions critical for adaptive behavior. A computation of the phase relationship between signals recorded from separable brain regions is a method for characterizing the temporal interactions of neuronal populations. Recently, different phase estimation methods for quantifying the time-varying and frequency-dependent nature of neural synchronization have been proposed. The most common method for measuring the synchronization of signals through phase computations uses complex wavelet transforms of neural signals to estimate their instantaneous phase difference and locking. In this article, we extend this idea by introducing a new time-varying phase synchrony measure based on Cohen's class of time-frequency distributions. This index offers improvements over existing synchrony measures by characterizing the similarity of signals from separable brain regions with uniformly high resolution across time and frequency. The proposed measure is applied to both synthesized signals and electroencephalography data to test its effectiveness in estimating phase changes and quantifying neural synchrony in the brain.
Hum Brain Mapp, 2010. © 2010 Wiley-Liss, Inc.
Figures

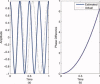
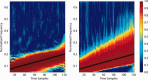
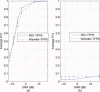
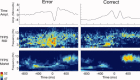
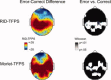
 computed with a Choi‐Williams kernel,
computed with a Choi‐Williams kernel,  , where σ = 0.001. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
, where σ = 0.001. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]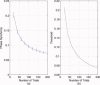
References
-
- Baccalá LA, Sameshima K ( 2001): Partial directed coherence: A new concept in neural structure determination. Biol Cybern 84: 463–474. - PubMed
-
- Breakspear M, Terry JR ( 2002): Nonlinear interdependence in neural systems: Motivation, theory, and relevance. Int J Neurosci 112: 1263–1284. - PubMed
-
- Brown P ( 2003): Oscillatory nature of human basal ganglia activity: Relationship to the pathophysiology of Parkinson's disease. Mov Disord 18: 357–363. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

