Detection of human polyomavirus proteins, T-antigen and agnoprotein, in human tumor tissue arrays
- PMID: 20336718
- PMCID: PMC2861297
- DOI: 10.1002/jmv.21514
Detection of human polyomavirus proteins, T-antigen and agnoprotein, in human tumor tissue arrays
Abstract
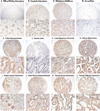
Expression of the human polyomavirus JCV genome in several experimental animals induces a variety of neural origin tumors. The viral proteins, T-antigen and Agnoprotein, contribute to the oncogenesis of JCV by associating with several tumor suppressor proteins and dysregulating signaling pathways, which results in uncontrolled cell proliferation. In addition, T-antigen and Agnoprotein have been associated with DNA damage and interfering with DNA repair mechanisms. In this study, we have utilized commercially available tissue arrays of human tumors of various origins and demonstrated the expression of both T-antigen and Agnoprotein in some, but not all, tumors of neural and non-neural origin. Most notably, more than 40% of human glioblastomas and greater than 30% of colon adenocarcinomas express viral proteins. The detection of viral transforming proteins, T-antigen and Agnoprotein in the absence of viral capsid proteins suggests a role for JCV in the development and/or progression of human tumors. These results invite further large-scale investigation on the role of polyomaviruses, particularly JCV in the pathogenesis of human cancer.
(c) 2010 Wiley-Liss, Inc.
Figures

References
-
- Behzad-Behbahani A, Klapper PE, Vallely PJ, Cleator GM, Khoo SH. Detection of BK virus and JC virus DNA in urine samples from immunocompromised (HIV-infected) and immunocompetent (HIV-non-infected) patients using polymerase chain reaction and microplate hybridization. J Clin Virol. 2004;29:224–229. - PubMed
-
- Bendiksen S, Rekvig OP, Van Ghelue M, Moens U. VP1 DNA sequences of JC and BK viruses detected in urine of systemic lupus erythematosus patients reveal no differences from strains expressed in normal individuals. J Gen Virol. 2000;81:2625–2633. - PubMed
-
- Ben-Ezra J, Johnson DA, Rossi J, Cook N, Wu A. Effect of fixation on the amplification of nucleic acids from paraffin-embedded material by the polymerase chain reaction. J Histochem Cytochem. 1991;39:351–354. - PubMed
-
- Berger JR, Concha M. Progressive multifocal leukoencephalopathy: The evolution of a disease once considered rare. J Neurovirol. 1995;1:5–18. - PubMed
-
- Bhattacharrya R, Noch EK, Khalili K. A novel role of Rac1 GTPase in JCV T-antigen mediated β-catenin stabilization. Oncogene. 2007;26:7628–7636. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

