Fast photochemical oxidation of protein footprints faster than protein unfolding
- PMID: 20337372
- PMCID: PMC3164994
- DOI: 10.1021/ac901054w
Fast photochemical oxidation of protein footprints faster than protein unfolding
Abstract
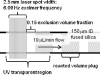
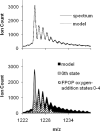
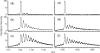
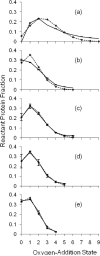
Fast photochemical oxidation of proteins (FPOP) is a chemical footprinting method whereby exposed amino-acid residues are covalently labeled by oxidation with hydroxyl radicals produced by the photolysis of hydrogen peroxide. Modified residues can be detected by standard trypsin proteolysis followed by LC/MS/MS, providing information about solvent accessibility at the peptide and even the amino-acid level. Like other chemical footprinting techniques, FPOP must ensure only the native conformation is labeled. Although oxidation via hydroxyl radical induces unfolding in proteins on a time scale of milliseconds or longer, FPOP is designed to limit (*)OH exposure to 1 micros or less by employing a pulsed laser for initiation to produce the radicals and a radical-scavenger to limit their lifetimes. We applied FPOP to three oxidation-sensitive proteins and found that the distribution of modification (oxidation) states is Poisson when a scavenger is present, consistent with a single conformation protein modification model. This model breaks down when a scavenger is not used and/or hydrogen peroxide is not removed following photolysis. The outcome verifies that FPOP occurs on a time scale faster than conformational changes in these proteins.
Figures

References
-
- Hirs CHW, Di Sabato G, Ottesen M, Gold AM, Gurd FRN, Horton HR, Koshland DE, Kimmel JR, Klotz IM, Ludwig ML, Hunter MJ, Neumann NP, Ray WJ, Riordan JF, Vallee BL, Sela M, Arnon R, Spande TF, Witkop B, Stark GR, Wilcox PE, Wold F. Methods in Enzymology. Vol. 11. Academic Press; 1967. pp. 485–748.
-
- Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Science. 1989;246:64–71. - PubMed
-
- Karas M, Bachmann D, Hillenkamp F. Anal. Chem. 1985;57:2935–2939.
-
- Lars Konermann XTYP. J. Mass Spectrom. 2008;43:1021–1036. - PubMed
-
- Xu G, Chance MR. Anal. Chem. 2004;76:1213–1221. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

