Novel metals and metal complexes as platforms for cancer therapy
- PMID: 20337575
- PMCID: PMC3759287
- DOI: 10.2174/138161210791209009
Novel metals and metal complexes as platforms for cancer therapy
Abstract
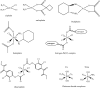
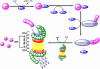
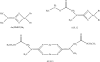
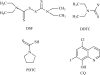
Metals are essential cellular components selected by nature to function in several indispensable biochemical processes for living organisms. Metals are endowed with unique characteristics that include redox activity, variable coordination modes, and reactivity towards organic substrates. Due to their reactivity, metals are tightly regulated under normal conditions and aberrant metal ion concentrations are associated with various pathological disorders, including cancer. For these reasons, coordination complexes, either as drugs or prodrugs, become very attractive probes as potential anticancer agents. The use of metals and their salts for medicinal purposes, from iatrochemistry to modern day, has been present throughout human history. The discovery of cisplatin, cis-[Pt(II) (NH(3))(2)Cl(2)], was a defining moment which triggered the interest in platinum(II)- and other metal-containing complexes as potential novel anticancer drugs. Other interests in this field address concerns for uptake, toxicity, and resistance to metallodrugs. This review article highlights selected metals that have gained considerable interest in both the development and the treatment of cancer. For example, copper is enriched in various human cancer tissues and is a co-factor essential for tumor angiogenesis processes. However the use of copper-binding ligands to target tumor copper could provide a novel strategy for cancer selective treatment. The use of nonessential metals as probes to target molecular pathways as anticancer agents is also emphasized. Finally, based on the interface between molecular biology and bioinorganic chemistry the design of coordination complexes for cancer treatment is reviewed and design strategies and mechanisms of action are discussed.
Figures

References
-
- DeVita VT, Jr., Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68:8643–53. - PubMed
-
- Hambley TW, Hait WN. Is anticancer drug development heading in the right direction? Cancer Res. 2009;69:1259–62. - PubMed
-
- Neidle S, Thurston DE. Chemical approaches to the discovery and development of cancer therapies. Nat Rev Cancer. 2005;5:285–96. - PubMed
-
- Orvig C, Abrams MJ. Medicinal inorganic chemistry: introduction. Chem Rev. 1999;99:2201–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

