Interaction of connexin43 and protein kinase C-delta during FGF2 signaling
- PMID: 20338032
- PMCID: PMC2855512
- DOI: 10.1186/1471-2091-11-14
Interaction of connexin43 and protein kinase C-delta during FGF2 signaling
Abstract
Background: We have recently demonstrated that modulation of the gap junction protein, connexin43, can affect the response of osteoblasts to fibroblast growth factor 2 in a protein kinase C-delta-dependent manner. Others have shown that the C-terminal tail of connexin43 serves as a docking platform for signaling complexes. It is unknown whether protein kinase C-delta can physically interact with connexin43.
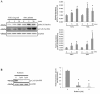
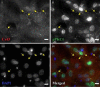
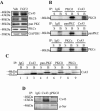
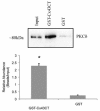
Results: In the present study, we investigate by immunofluorescent co-detection and biochemical examination the interaction between Cx43 and protein kinase C-delta. We establish that protein kinase C-delta physically interacts with connexin43 during fibroblast growth factor 2 signaling, and that protein kinase C delta preferentially co-precipitates phosphorylated connexin43. Further, we show by pull down assay that protein kinase C-delta associates with the C-terminal tail of connexin43.
Conclusions: Connexin43 can serve as a direct docking platform for the recruitment of protein kinase C-delta in order to affect fibroblast growth factor 2 signaling in osteoblasts. These data expand the list of signal molecules that assemble on the connexin43 C-terminal tail and provide a critical context to understand how gap junctions modify signal transduction cascades in order to impact cell function.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

