Mechanisms and significance of nuclear receptor auto- and cross-regulation
- PMID: 20338175
- PMCID: PMC2911511
- DOI: 10.1016/j.ygcen.2010.03.013
Mechanisms and significance of nuclear receptor auto- and cross-regulation
Abstract
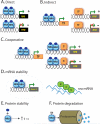
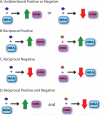
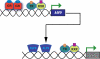
The number of functional hormone receptors expressed by a cell in large part determines its responsiveness to the hormonal signal. The regulation of hormone receptor gene expression is therefore a central component of hormone action. Vertebrate steroid and thyroid hormones act by binding to nuclear receptors (NR) that function as ligand-activated transcription factors. Nuclear receptor genes are regulated by diverse and interacting intracellular signaling pathways. Nuclear receptor ligands can regulate the expression of the gene for the NR that mediates the hormone's action (autoregulation), thus influencing how a cell responds to the hormone. Autoregulation can be either positive or negative, the hormone increasing or decreasing, respectively, the expression of its own NR. Positive autoregulation (autoinduction) is often observed during postembryonic development, and during the ovarian cycle, where it enhances cellular sensitivity to the hormonal signal to drive the developmental process. By contrast, negative autoregulation (autorepression) may become important in the juvenile and adult for homeostatic negative feedback responses. In addition to autoregulation, a NR can influence the expression other types of NRs (cross-regulation), thus modifying how a cell responds to a different hormone. Cross-regulation by NRs is an important means to temporally coordinate cell responses to a subsequent (different) hormonal signal, or to allow for crosstalk between hormone signaling pathways.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
References
-
- Alexander IE, Clarke CL, Shine J, Sutherland RL. Progestin inhibition of progesterone receptor gene expression in human breast cancer cells. Mol. Endocrinol. 1989;3:1377–1386. - PubMed
-
- Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol. Rev. 2001;81:1269–1304. - PubMed
-
- Arbour NC, Prahl JM, Deluca HF. Stabilization of the vitamin-D receptor in rat osteosarcoma cells through the action of 1,25-dihydroxyvitamin-D(3). Mol. Endocrinol. 1993;7:1307–1312. - PubMed
-
- Bagamasbad P, Howdeshell KL, Sachs LM, Demeneix BA, Denver RJ. A role for basic transcription element-binding protein 1 (BTEB1) in the autoinduction of thyroid hormone receptor beta. J. Biol. Chem. 2008;283:2275–2285. - PubMed
-
- Baker BS, Tata JR. Prolactin prevents the autoinduction of thyroid hormone receptor mRNAs during amphibian metamorphosis. Dev. Biol. 1992;149:463–467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

