Molecular resurfacing of cartilage with proteoglycan 4
- PMID: 20338268
- PMCID: PMC2908720
- DOI: 10.1016/j.actbio.2010.03.025
Molecular resurfacing of cartilage with proteoglycan 4
Abstract
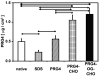
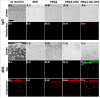
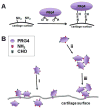
Early loss of proteoglycan 4 (PRG4), a lubricating glycoprotein implicated in boundary lubrication, from the cartilage surface has been associated with degeneration of cartilage and early onset of osteoarthritis. Viscosupplementation with hyaluronic acid and other macromolecules has been proposed as a treatment of osteoarthritis. However, the efficacy of viscosupplementation is variable and may be influenced by the short residence time of lubricant in the knee joint after injection. Recent studies have demonstrated the use of aldehyde (CHO) modified extracellular matrix proteins for targeted adherence to a biological tissue surface. It is hypothesized that CHO could be exploited to enhance the binding of lubricating proteoglycans to the surface of PRG4-depleted cartilage. The objective of this study was to determine the feasibility of molecular resurfacing of cartilage with CHO-modified PRG4. PRG4 was chemically functionalized with aldehyde (PRG4-CHO) and aldehyde plus Oregon Green (OG) fluorophore (PRG4-OG-CHO) to allow for differentiation of endogenous and exogenous PRG4. Cartilage disks depleted of native PRG4 were then treated with solutions of PRG4, PRG4-CHO, or PRG4-OG-CHO and then assayed for the presence of PRG4 by immunohistochemistry, ELISA, and fluorescence imaging. Repletion of cartilage surfaces was significantly enhanced with the inclusion of CHO compared with repletion with unmodified PRG4. These findings suggest a generalized approach which may be used for molecular resurfacing of tissue surfaces with PRG4 and other lubricating biomolecules, perhaps leading in the future to a convenient method for overcoming loss of lubrication during the early stages of osteoarthritis.
Published by Elsevier Ltd.
Figures
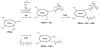


References
-
- Schinagl RM, Gurskis D, Chen AC, Sah RL. Depth-dependent confined compression modulus of full-thickness bovine articular cartilage. J Orthop Res. 1997;15:499. - PubMed
-
- Schumacher BL, Su J-L, Lindley KM, Kuettner KE, Cole AA. Horizontally oriented clusters of multiple chondrons in the superficial zone of ankle, but not knee articular cartilage. Anat Rec. 2002;266:241. - PubMed
-
- Jadin KD, Wong BL, Bae WC, Li KW, Williamson AK, Schumacher BL, Price JH, Sah RL. Depth-varying density and organization of chondrocyte in immature and mature bovine articular cartilage assessed by 3-D imaging and analysis. J Histochem Cytochem. 2005;53:1109. - PubMed
-
- Buckwalter JA, Mankin HJ. Articular cartilage. Part II: degeneration and osteoarthrosis, repair, regeneration, and transplantation. J Bone Joint Surg Am. 1997;79-A:612.
-
- Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64-A:460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

