Sodium perchlorate effects on the helical stability of a mainly alanine peptide
- PMID: 20338840
- PMCID: PMC2808484
- DOI: 10.1016/j.bpj.2009.10.013
Sodium perchlorate effects on the helical stability of a mainly alanine peptide
Erratum in
- Biophys J. 2010 Oct 20;99(8):2715. Xiong, Kang [corrected to Xiong, Kan]
Abstract
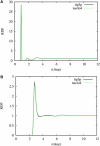
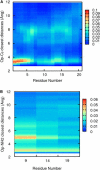
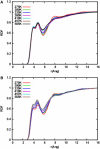
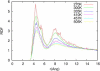
Sodium perchlorate salt (NaClO(4)) is commonly used as an internal intensity standard in ultraviolet resonance Raman (UVRR) spectroscopy experiments. It is well known that NaClO(4) can have profound effects on peptide stability. The impact of NaClO(4) on protein stability in UVRR experiments has not yet been fully investigated. It is well known from experiment that protein stability is strongly affected by the solution composition (water, salts, osmolytes, etc.). Therefore, it is of the utmost importance to understand the physical basis on which the presence of salts and osmolytes in the solution impact protein structure and stability. The aim of this study is to investigate the effects of NaClO(4), on the helical stability of an alanine peptide in water. Based upon replica-exchange molecular dynamics data, it was found that NaClO(4) solution strongly stabilizes the helical state and that the number of pure helical conformations found at room temperature is greater than in pure water. A thorough investigation of the anion effects on the first and second solvation shells of the peptide, along with the Kirkwood-Buff theory for solutions, allows us to explain the physical mechanisms involved in the observed specific ion effects. A direct mechanism was found in which ClO(4)(-) ions are strongly attracted to the folded backbone.
Copyright 2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Hofmeister F. Zur Lehre von der Wirkung der Salze. Arch. Exp. Pathol. Pha. 1888;24:247–260.
-
- Conway B.E. Elsevier; Amsterdam, The Netherlands: 1981. Ionic Hydration in Chemistry and Biophysics.
-
- Edsall J.T., McKenzie H.A. Water and proteins. II. The location and dynamics of water in protein systems and its relation to their stability and properties. Adv. Biophys. 1983;16:53–183. - PubMed
-
- Cappa C.D., Smith J.D., Saykally R.J. Effects of alkali metal halide salts on the hydrogen bond network of liquid water. J. Phys. Chem. B. 2005;109:7046–7052. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

