Motivating the unmotivated for health behavior change: a randomized trial of cessation induction for smokers
- PMID: 20338901
- PMCID: PMC2902976
- DOI: 10.1177/1740774510361533
Motivating the unmotivated for health behavior change: a randomized trial of cessation induction for smokers
Abstract
Background: Many smokers remain unwilling or unable to make a quit attempt. For these smokers, novel strategies to induce quit attempts are necessary to achieve further reductions in smoking prevalence.
Purpose: This article describes the design and methods of an ongoing nationwide telephone-based clinical trial for cessation induction, the principal aim of which is to test the hypothesis that samples of nicotine replacement therapy (NRT), can induce quit attempts among smokers otherwise unmotivated to quit.
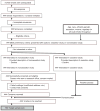
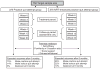
Methods: Smokers are recruited proactively through online channels. A 'behavioral filter' is used to identify and separate motivated versus unmotivated smokers, the latter of whom (N = 750) are formally entered into the clinical trial. Participants are randomized to one of two treatment conditions designed to promote self-efficacy and motivation to quit: (1) practice quit attempt (PQA) or (2) PQA plus NRT sampling. The primary outcome measure tested over a 6-month follow-up is the incidence of additional quit attempts as well as hypothesized mediators of treatment effects.
Results: This study details the challenges of identifying and treating smokers who are unmotivated to quit. Strengths include a novel treatment approach, tested among a group of proactively recruited smokers nationwide, with a unique method of identifying cessation-resistant smokers.
Limitations: The omission of a true control group, testing the effect of the PQA itself, is an inherent limitation to the study design. Online recruitment presents additional study challenges, all of which are discussed in detail.
Conclusions: The study has translational potential to guide both clinical and policy recommendations for cessation induction. Further, while the focus is on smoking, this trial may serve as an example to researchers and clinicians who focus on other health behaviors, and who themselves are challenged with motivating people who are unmotivated for change.
Figures
References
-
- Wewers ME, Stillman FA, Hartman AM, Shopland DR. Distribution of daily smokers by stage of change: Current Population Survey results. Prev Med. 2003;36:710–20. - PubMed
-
- USDHS. Cigarette smoking among adults — United States, 2006. MMWR. 2007;56:1157–61. - PubMed
-
- Stead LF, Bergson G, Lancaster T. The Cochrane Library. 3. Wiley; Oxford: 2008. Physician Advice for Smoking Cessation (Cochrane Review) - PubMed
-
- Fiore MC, Jaén CR, Baker TB, et al. Clinical Practice Guideline. US Public Health Service; Rockville, MD: 2008. Treating Tobacco Use and Dependence: 2008 Update.
-
- Prochaska JO, Redding CA, Evers KE. The transtheoretical model and stages of change. In: Glanz K, RImer B, Lewis F, editors. Health Behavior and Health Education: Theory, Research, and Practice. Jossey-Bass; San Francisco: 2002. pp. 99–120.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical