mTOR complex-2 activates ENaC by phosphorylating SGK1
- PMID: 20338997
- PMCID: PMC2865740
- DOI: 10.1681/ASN.2009111168
mTOR complex-2 activates ENaC by phosphorylating SGK1
Abstract
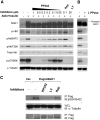
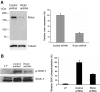
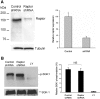
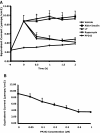
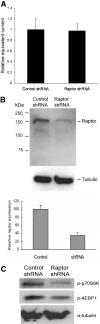
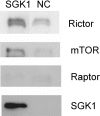
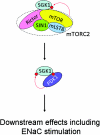
The serum- and glucocorticoid-induced kinase 1 (SGK1) plays a central role in hormone regulation of epithelial sodium (Na+) channel (ENaC)-dependent Na+ transport in the distal nephron. Phosphorylation within a carboxy-terminal domain, designated the hydrophobic motif (HM), determines the activity of SGK1, but the identity of the HM kinase is unknown. Here, we show that the highly conserved serine-threonine kinase mammalian target of rapamycin (mTOR) is essential for the phosphorylation of the HM of SGK1 and the activation of ENaC. We observed that mTOR, in conjunction with rictor (mTORC2), phosphorylated SGK1 and stimulated ENaC. In contrast, when mTOR assembled with raptor in the rapamycin-inhibited complex (mTORC1), it did not phosphorylate SGK1 or stimulate ENaC. Inhibition of mTOR blocked both SGK1 phosphorylation and ENaC-mediated Na+ transport, whereas specific inhibition of mTORC1 had no effect. Similarly, small hairpin RNA-mediated knockdown of rictor inhibited SGK1 phosphorylation and Na+ current, whereas knockdown of raptor had no effect. Finally, in co-immunoprecipitation experiments, SGK1 interacted selectively with rictor but not with raptor, suggesting selective recruitment of SGK1 to mTORC2. We conclude that mTOR, specifically mTORC2, is the HM kinase for SGK1 and is required for ENaC-mediated Na+ transport, thereby extending our understanding of the molecular mechanisms underlying Na+ balance.
Figures
Similar articles
-
mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1).Biochem J. 2008 Dec 15;416(3):375-85. doi: 10.1042/BJ20081668. Biochem J. 2008. PMID: 18925875
-
Insulin upregulates the expression of epithelial sodium channel in vitro and in a mouse model of acute lung injury: role of mTORC2/SGK1 pathway.Exp Cell Res. 2015 Feb 1;331(1):164-175. doi: 10.1016/j.yexcr.2014.09.024. Epub 2014 Sep 28. Exp Cell Res. 2015. PMID: 25265063
-
Regulation of the epithelial Na+ channel by the mTORC2/SGK1 pathway.Nephrol Dial Transplant. 2016 Feb;31(2):200-5. doi: 10.1093/ndt/gfv270. Epub 2015 Jul 9. Nephrol Dial Transplant. 2016. PMID: 26163195 Free PMC article. Review.
-
mTORC2 regulates renal tubule sodium uptake by promoting ENaC activity.J Clin Invest. 2015 Jan;125(1):117-28. doi: 10.1172/JCI73935. Epub 2014 Nov 21. J Clin Invest. 2015. PMID: 25415435 Free PMC article.
-
New insights into the role of serum- and glucocorticoid-inducible kinase SGK1 in the regulation of renal function and blood pressure.Curr Opin Nephrol Hypertens. 2005 Jan;14(1):59-66. doi: 10.1097/00041552-200501000-00010. Curr Opin Nephrol Hypertens. 2005. PMID: 15586017 Review.
Cited by
-
Airway surface hyperviscosity and defective mucociliary transport by IL-17/TNF-α are corrected by β-adrenergic stimulus.JCI Insight. 2022 Nov 22;7(22):e164944. doi: 10.1172/jci.insight.164944. JCI Insight. 2022. PMID: 36219481 Free PMC article.
-
Role of epithelial sodium channels and their regulators in hypertension.J Biol Chem. 2010 Oct 1;285(40):30363-9. doi: 10.1074/jbc.R110.155341. Epub 2010 Jul 12. J Biol Chem. 2010. PMID: 20624922 Free PMC article. Review.
-
AKT-independent PI3-K signaling in cancer - emerging role for SGK3.Cancer Manag Res. 2013 Aug 26;5:281-92. doi: 10.2147/CMAR.S35178. eCollection 2013. Cancer Manag Res. 2013. PMID: 24009430 Free PMC article. Review.
-
Proximal tubular epithelial insulin receptor mediates high-fat diet-induced kidney injury.JCI Insight. 2021 Feb 8;6(3):e143619. doi: 10.1172/jci.insight.143619. JCI Insight. 2021. PMID: 33400689 Free PMC article.
-
An unyielding challenge for refractory hyponatremia in neuroendocrine cervix carcinoma: a case report.Front Endocrinol (Lausanne). 2025 Feb 28;16:1451157. doi: 10.3389/fendo.2025.1451157. eCollection 2025. Front Endocrinol (Lausanne). 2025. PMID: 40093752 Free PMC article.
References
-
- Wullschleger S, Loewith R, Hall MN: TOR signaling in growth and metabolism. Cell 124: 471–484, 2006. - PubMed
-
- Guertin DA, Sabatini DM: Defining the role of mTOR in cancer. Cancer Cell 12: 9–22, 2007. - PubMed
-
- Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, Tokunaga C, Avruch J, Yonezawa K: Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110: 177–189, 2002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

