eIF2alpha phosphorylation tips the balance to apoptosis during osmotic stress
- PMID: 20338999
- PMCID: PMC2878040
- DOI: 10.1074/jbc.M110.109439
eIF2alpha phosphorylation tips the balance to apoptosis during osmotic stress
Abstract
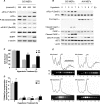
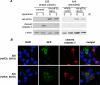
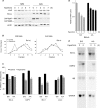
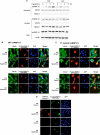
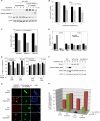
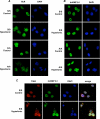
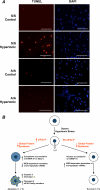
Regulation of cell volume is of great importance because persistent swelling or shrinkage leads to cell death. Tissues experience hypertonicity in both physiological (kidney medullar cells) and pathological states (hypernatremia). Hypertonicity induces an adaptive gene expression program that leads to cell volume recovery or apoptosis under persistent stress. We show that the commitment to apoptosis is controlled by phosphorylation of the translation initiation factor eIF2alpha, the master regulator of the stress response. Studies with cultured mouse fibroblasts and cortical neurons show that mutants deficient in eIF2alpha phosphorylation are protected from hypertonicity-induced apoptosis. A novel link is revealed between eIF2alpha phosphorylation and the subcellular distribution of the RNA-binding protein heterogeneous nuclear ribonucleoprotein A1 (hnRNP A1). Stress-induced phosphorylation of eIF2alpha promotes apoptosis by inducing the cytoplasmic accumulation of hnRNP A1, which attenuates internal ribosome entry site-mediated translation of anti-apoptotic mRNAs, including Bcl-xL that was studied here. Hypertonic stress induced the eIF2alpha phosphorylation-independent formation of cytoplasmic stress granules (SGs, structures that harbor translationally arrested mRNAs) and the eIF2alpha phosphorylation-dependent accumulation of hnRNP A1 in SGs. The importance of hnRNP A1 was demonstrated by induction of apoptosis in eIF2alpha phosphorylation-deficient cells that express exogenous cytoplasmic hnRNP A1. We propose that eIF2alpha phosphorylation during hypertonic stress promotes apoptosis by sequestration of specific mRNAs in SGs in a process mediated by the cytoplasmic accumulation of hnRNP A1.
Figures
References
-
- Burg M. B., Ferraris J. D., Dmitrieva N. I. (2007) Physiol. Rev. 87, 1441–1474 - PubMed
-
- Maallem S., Mutin M., González-González I. M., Zafra F., Tappaz M. L. (2008) Neuroscience 153, 95–107 - PubMed
-
- Loyher M. L., Mutin M., Woo S. K., Kwon H. M., Tappaz M. L. (2004) Neuroscience 124, 89–104 - PubMed
-
- Lin M., Liu S. J., Lim I. T. (2005) Emerg. Med. Clin. North Am. 23, 749–770, ix - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL057346/HL/NHLBI NIH HHS/United States
- R01 DK053307/DK/NIDDK NIH HHS/United States
- DK42304/DK/NIDDK NIH HHS/United States
- R37 DK042394/DK/NIDDK NIH HHS/United States
- FRN 74740/PHS HHS/United States
- DK53307/DK/NIDDK NIH HHS/United States
- R01 DK060596/DK/NIDDK NIH HHS/United States
- MOP 89737/PHS HHS/United States
- R01 HL052173/HL/NHLBI NIH HHS/United States
- HL057346/HL/NHLBI NIH HHS/United States
- DK60596/DK/NIDDK NIH HHS/United States
- R01 DK088227/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- HL52173/HL/NHLBI NIH HHS/United States
- R37 DK060596/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

