Common structural motifs for the regulation of divergent class II myosins
- PMID: 20339003
- PMCID: PMC2878022
- DOI: 10.1074/jbc.R109.025551
Common structural motifs for the regulation of divergent class II myosins
Abstract
This minireview focuses on structural studies that have provided insights into our current understanding of thick filament regulation in muscle. We describe how different domains in the myosin molecule interact to produce an inactive "off" state; included are head-head and head-rod interactions, the role of the regulatory light chain, and the significance of the alpha-helical coiled-coil rod in regulation. Several of these interactions have now been visualized in a wide variety of native myosin filaments, testifying to the generality of these structural motifs across the phylogenetic tree.
Figures

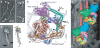
References
-
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. (1993) Science 261, 50–58 - PubMed
-
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. (1993) Science 261, 58–65 - PubMed
-
- Adelstein R. S., Conti M. A. (1975) Nature 256, 597–598 - PubMed
-
- Adelstein R. S., Eisenberg E. (1980) Annu. Rev. Biochem. 49, 921–956 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

